Cyclobenzaprine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- CYCLOBENZAPRINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CYCLOBENZAPRINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CYCLOBENZAPRINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
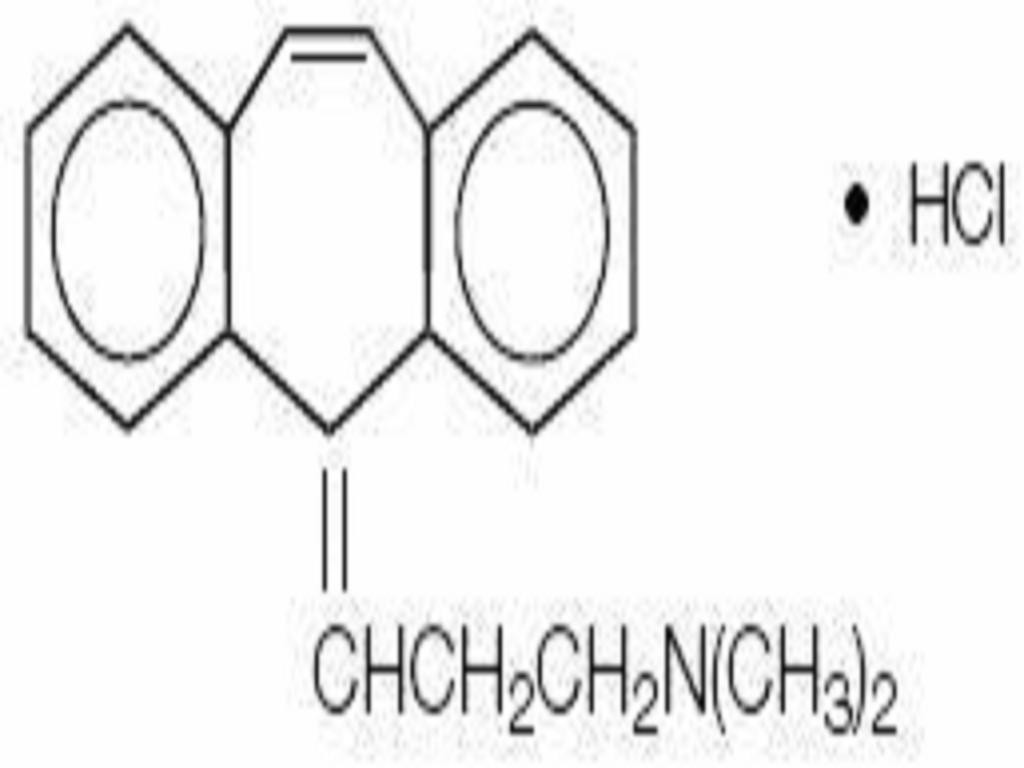
CYCLOBENZAPRINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Pharmacokinetics
PRECAUTIONS, Use in the ElderlyPRECAUTIONS, Impaired Hepatic Function
Elderly
Hepatic Impairment
Clinical Studies
Surveillance Program
ADVERSE REACTIONS
INDICATIONS & USAGE
CYCLOBENZAPRINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
WARNINGSADVERSE REACTIONSPRECAUTIONS
GeneralImpaired Hepatic Function
CLINICAL PHARMACOLOGY, PharmacokineticsHepatic Impairment
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CONTRAINDICATIONSCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGY, PharmacokineticsElderlyCYCLOBENZAPRINE HYDROCHLORIDE ADVERSE REACTIONS
Clinical Studies withSurveillance Program withCyclobenzaprine HCl Tablets 10 mgCyclobenzaprine HCl Tablets 10 mg
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
ADVERSE REACTIONS
DOSAGE & ADMINISTRATION
INDICATIONS AND USAGEPRECAUTIONS, Impaired Hepatic FunctionUse in the Elderly
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Cyclobenzaprine HydrochlorideCyclobenzaprine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!