CVS Pharmacy Dandruff
CVS Pharmacy Inc
APOLLO HEALTH AND BEAUTY CARE
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- CVS Pharmacy Dandruff Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Package Front and Back Labels
FULL PRESCRIBING INFORMATION
Active Ingredient
Selenium Sulfide 1%
Purpose
Anti-Dandruff
CVS Pharmacy Dandruff Uses
Controls flaking, scaling and itching associated with dandruff
Warnings
For external use only
Do Not Use
- On scalp that is broken or inflamed.
- If you are allergic to ingredients in this product
When using this product
avoid contact with eyes. If contact occurs, rinse with water. For use on color treated or permed hair, rinse thoroughly
Stop using this product and ask doctor if
- rash appears
- condition worsens or does not improve in 2-4 weeks
Keep out of reach of children
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately
If pregnant or breast feeding
ask a doctor before use
Directions
- Adults and children 12 years and over: wet hair thoroughly, apply shampoo, generously lather, rinse thoroughly. Repeat. Use every 3-4 days for up to 8 weeks or as directed by a doctor. Then use only as needed to control dandruff.
-
Children under 12 years: ask a doctor
Other Information
- store between 35o and 85o F (2o and 30oC)
- protect from freezing
- see bottom of bottle for lot number and expiration date
Inactive Ingredients
Water (Aqua), Ammonium Lauryl Sulfate, Ammonium Laureth Sulfate, Dihydrogenated Tallow Phthalic Acid Amide, Cocamide DEA, Fragrance (Parfum), Titanium Dioxide, Dimethicone, Hydroxypropyl Methylcellulose, Citric Acid, Sodium Isostearoyl Lactylate, DMDM Hydantoin, Aloe Barbadensis Leaf Juice, Sodium Citrate, Sodium Chloride, Blue 1 (CI 42090)
Package Front and Back Labels
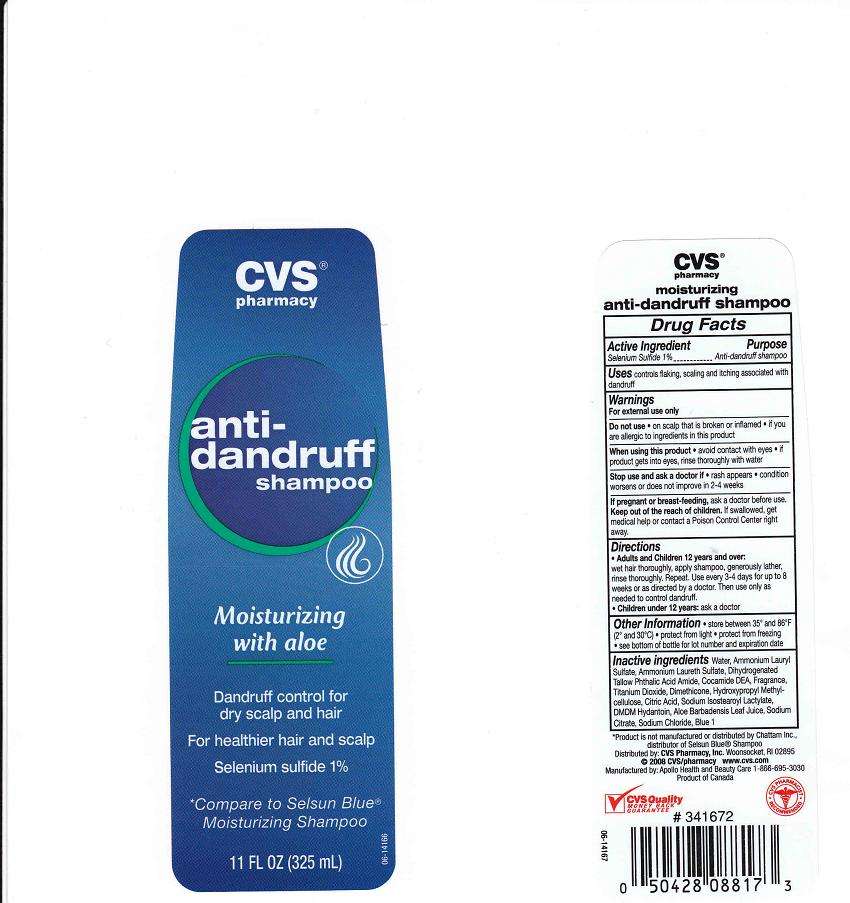
CVS Pharmacy DandruffSelenium Sulfide SHAMPOO
| |||||||||||||||||||||||||||||||||||||||||||||||||