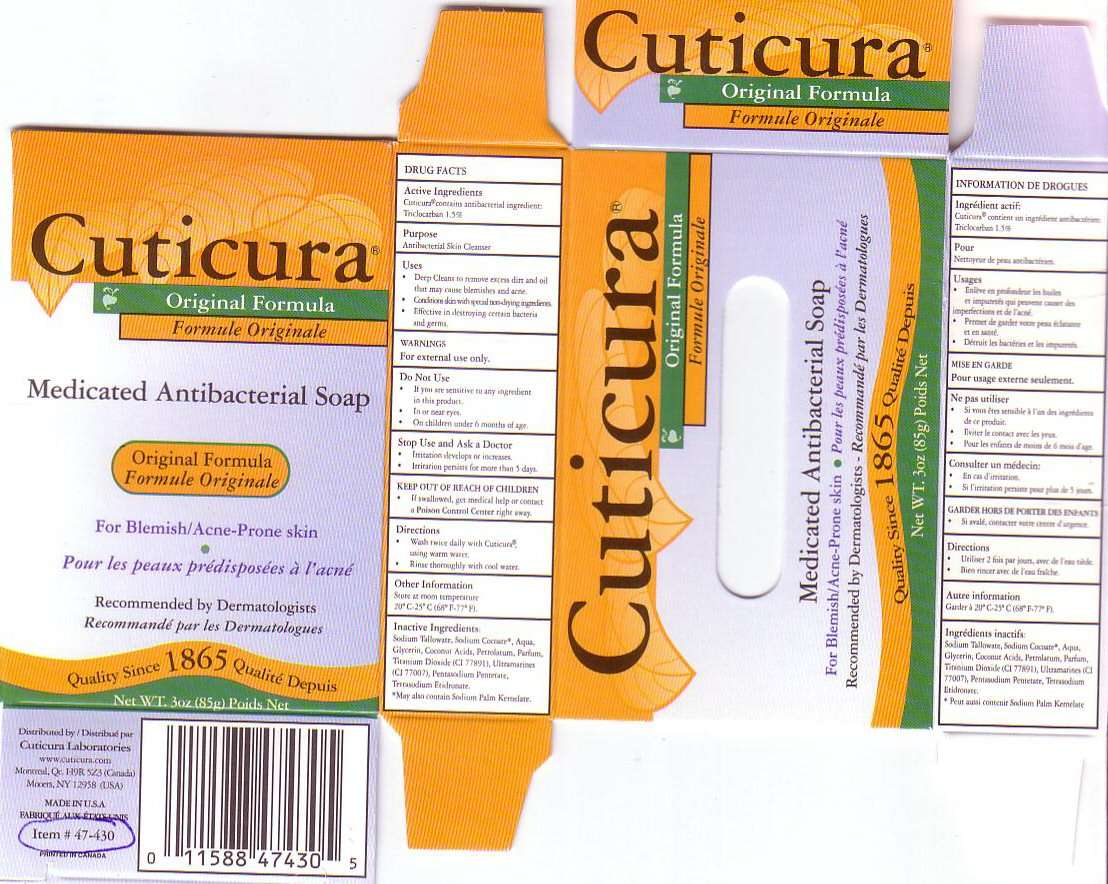
CUTICURA MEDICATED ANTIBACTERIAL BAR
Bradford Soap Works, Inc.
Bradford Soap Works, Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
- DO NOT USE
- STOP USE AND ASK A DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
CUTICURA CONTAINS ANTIBACTERIAL INGREDIENT: TRICLOCARBAN 1.5%
PURPOSE
ANTIBACTERIAL SKIN CLEANSER
USES
* DEEP CLEANS TO REMOVE EXCESS DIRT AND OIL THAT MAY CAUSE BLEMISHES AND ACNE.
* CONDITIONS SKIN WITH SPECIAL NON-DRYING INGREDIENTS.
* EFFECTIVE IN DESTROYING CERTAIN BACTERIA AND GERMS.
WARNINGS
FOR EXTERNAL USE ONLY
DO NOT USE
- IF YOU ARE SENSITIVE TO ANY INGREDIENTS IN THIS PRODUCT.
- IN OR NEAR EYES
- ON CHILDREN UNDER 6 MONTHS OF AGE.
STOP USE AND ASK A DOCTOR
- IRRITATION DEVELOPS OR INCREASES.
- IRRITATION PERSISTS FOR MORE THAN 5 DAYS.
KEEP OUT OF REACH OF CHILDREN
IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- WASH TWICE DAILY WITH CUTICURA, USING WARM WATER.
- RINSE THOROUGHLY WITH COOL WATER.
OTHER INFORMATION
STORE AT ROOM TEMPERATURE
200 C - 250 C (680 F - 770 F)
INACTIVE INGREDIENTS
SODIUM TALLOWATE, SODIUM COCOATE, WATER, GLYCERIN, COCONUT ACIDS, PETROLATUM, PARFUM, TITANIUM DIOXIDE (CI 77891), ULTRAMARINES (CI 7007), PENTASODIUM PENTETATE, TETRASODIUM ETIDRONATE.
*MAY ALSO CONTAIN SODIUM PALM KERNELATE.
CUTICURA ORIGINAL FORMULA MEDICATED ANTIBACTERIAL SOAP ORIGINAL FORMULA FOR BLEMISH / ACNE-PRONE SKIN RECOMMENDED BY DERMATOLOGISTS QUALITY SINCE 1865
CUTICURA MEDICATED ANTIBACTERIAL BARTRICLOCARBAN SOAP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||