CounterAct
CounterAct Day Content of Label
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients (in each caplet)
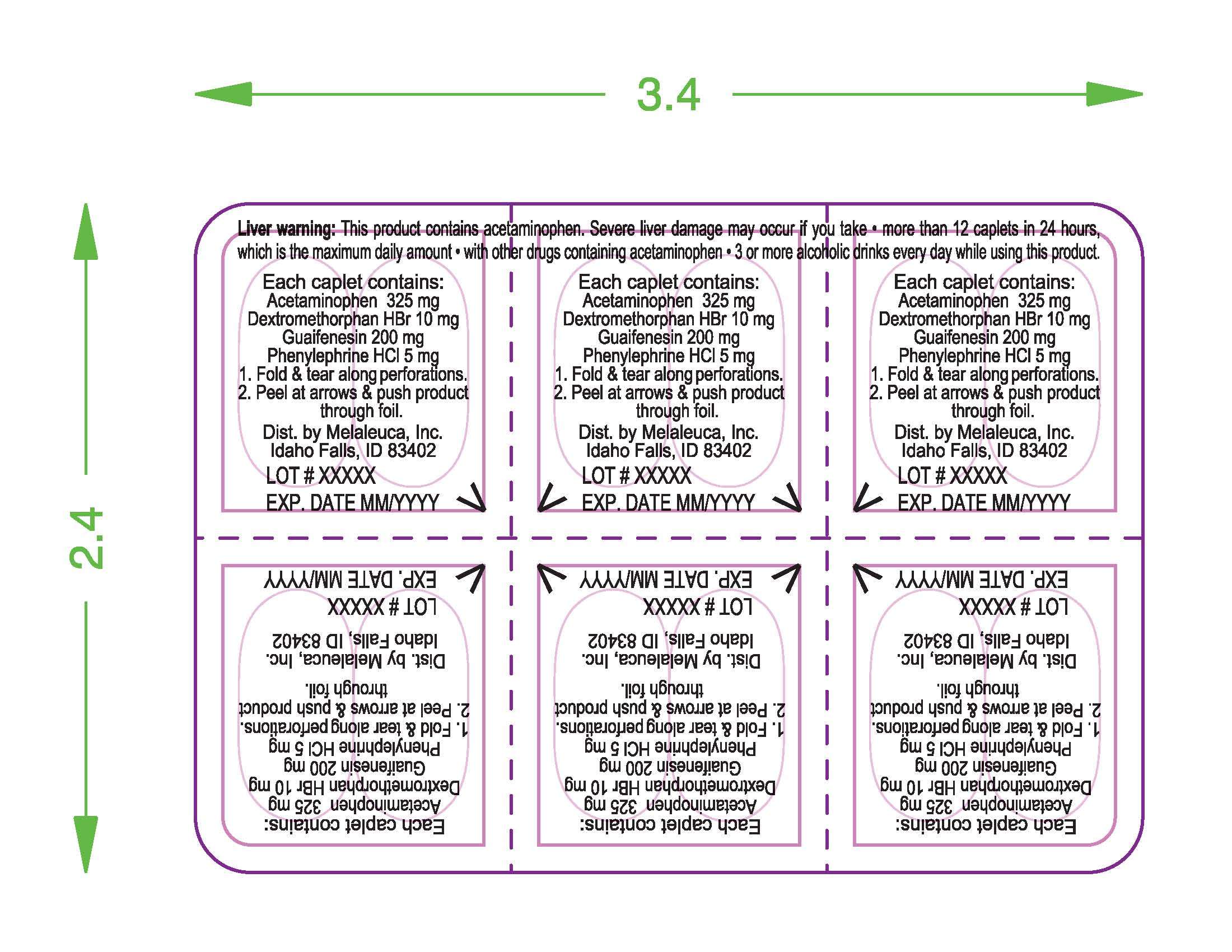
Acetaminophen 325 mg
Dextromethorphan HBr 10 mg
Guaifenesin 200 mg
Phenylephrine HCI 5 mg
Purpose
Purpose
Pain reliever/fever reducer
Cough suppressant
Expectorant
Nasal decongestant
Uses
Uses
- temporarily relieves these common cold/flu symptoms:
-
- headache
- minor aches and pains
- sore throat
- nasal congestion
- cough
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- diabetes
- heart disease
- trouble urinating due to and enlarged prostate gland
- high blood pressure
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- thyroid disease
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin.
When using this product do not exceed recommended dosage.
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- new symptoms occur
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- cough comes back or occurs with rash or headaches that lasts.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children, even if you do not notice any signs or symptoms.
Directions
- do not take more than directed (see overdose warning)
- adults and children 12 years and over
-
- take 2 caplets every 4 hours
- swallow whole - do not crush, chew, or dissolve
- do not take more than 12 caplets in 24 hours
- children under 12 years: do not use this adult product in children under 12 years of age; this will provide more than the recommended dose (overdose) and may cause liver damage
Other information
- store at controlled room temperature 15°-30°C (59°-86°F)
Inactive ingredients croscarmellose sodium, crospovidone, D and C yellow number 10 lake, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, silicon dioxide, sodium starch glycolate, stearic acid, titanium dioxide
Questions or comments? 1-800-426-9391
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN, OR SHOWS ANY SIGNS OF TAMPERING

CounterActAcetaminophen, Dextromethorphan HBr, Guaifenesin, Phenylephrine TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||