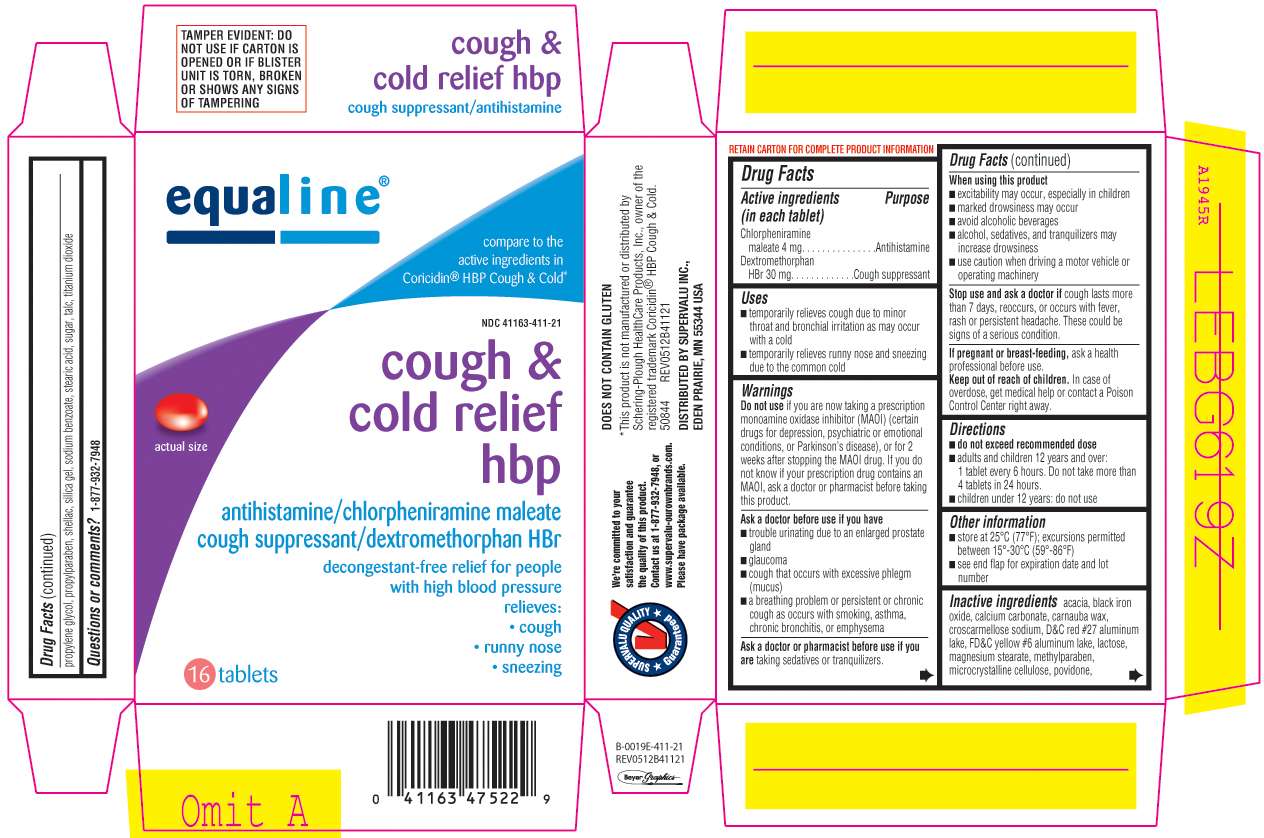
Cough and Cold Relief HBP
Equaline 44-411
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Chlorpheniramine maleate 4 mg
Dextromethorphan HBr 30 mg
Antihistamine
Cough suppressant
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- temporarily relieves runny nose and sneezing due to the common cold
Do not use
if you are taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- trouble urinating due to an enlarged prostate gland
- glaucoma
- cough that occurs with excessive phlegm (mucus)
- a breathing problem or presistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers.
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives, and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
cough lasts more than 7 days, reoccurs with fever, rash or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
- do not exceed recommended dose
- adults and children 12 years and over: 1 tablet every 6 hours. Do not take more than 4 tablets in 24 hours
- children under 12 years: do not use
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
acacia, black iron oxide, calcium carbonate, carnauba was, croscarmellose sodium, D&C red #27 aluminum lake, FD&C yellow #6 aluminum lake, lactose, magnesium searate, methylparaben, microcrystalline cellulose, povidone, propylparaben, propylene glycol, shellac, silica gel, sodium benzoate, stearic acid, sugar, talc, titanium dioxide
Principal Display Panel
equaline®
compare to the active ingredients Coricidin® HBP Cough & Cold*
NDC 41163-411-21
cough &
cold relief
hbp
antihistamine/chlropheniramine maleate
cough suppressant/dextromethorphan HBr
decongestant-free relief for people
with high blood pressure
relieves:
• cough
• runny nose
• sneezing
16 tablets
*This product is not manufactured or distributed by Schering-Plough HealthCare Products, Inc., owner of the registered trademark Coricidin® HBP Cough & Cold.
50844 REV0512B41121
DISTRIBUTED BY SUPERVALU INC.
EDEN PRARIE, MN 55344 USA
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Cough and Cold Relief HBPChlorpheniramine maleate and Dextromethorphan HBr TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||