Copper Mate
Fondel Chemical Ltd.
Fondel Chemical Ltd.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Copper Mate Drug Facts Label
Active Ingredients: Cupric Sulfate, Zinc Sulfate, Citric Acid [Powder for use in solution]
Directions: It is recommended for use in a 1% to 3% solution strength (by weight) with immersion lasting between 5 and 20 minutes, once or twice daily, for a period of time as prescribed by your veterinarian.
Warnings:
- Copper and Zinc can be toxic to sheep. Do not allow animals to eat or drink the Copper Mate powder or hoof bath solution.
- Know the volume of the hoof bath and calculate the amount of Copper Mate carefully.
- DO NOT OVER DOSE by using more Copper Mate than what is recommended by your veterinarian.
Keep out of reach of children.
Purpose
Purpose: Copper Mate powder is used as an aid in hoof rot management under veterinary guidance.
Use: Copper Mate powder is used in hoof baths for cattle and sheep.
When Using this Product: By placing a clean water bath ahead of the treatment bath, animals will clean their hooves to some extent and keep the treatment bath clean longer. Hoof baths should only be part of an overall program that includes proper nutrition, regular hoof trimming, and hoof injury prevention.
Questions?: 1-800-567-7455 (24/7-Emergency Spill Line) or 1-905-878-8432
Copper Mate Bag Label
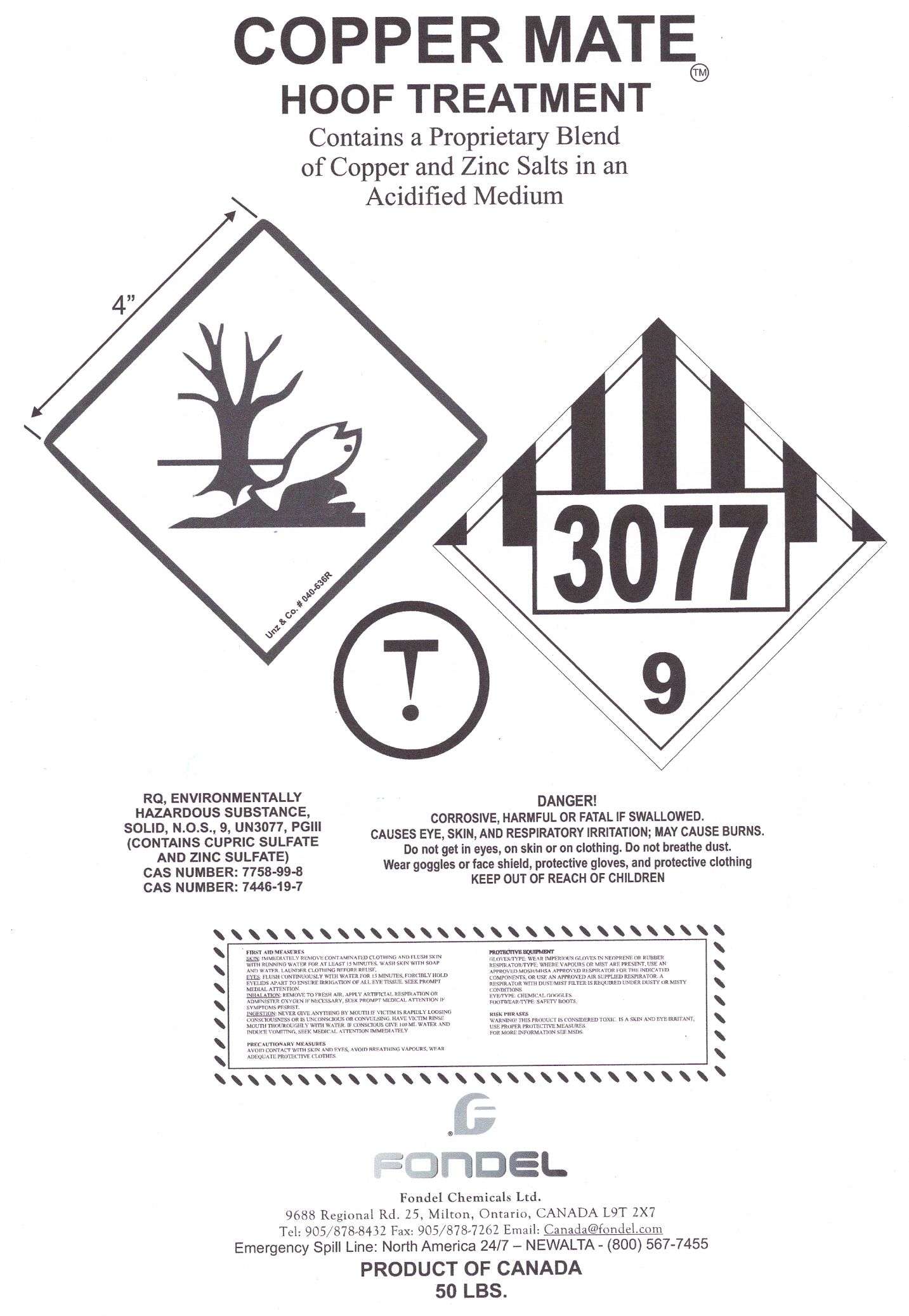
Copper MateAcidified Cupric and Zinc Sulfate POWDER, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||