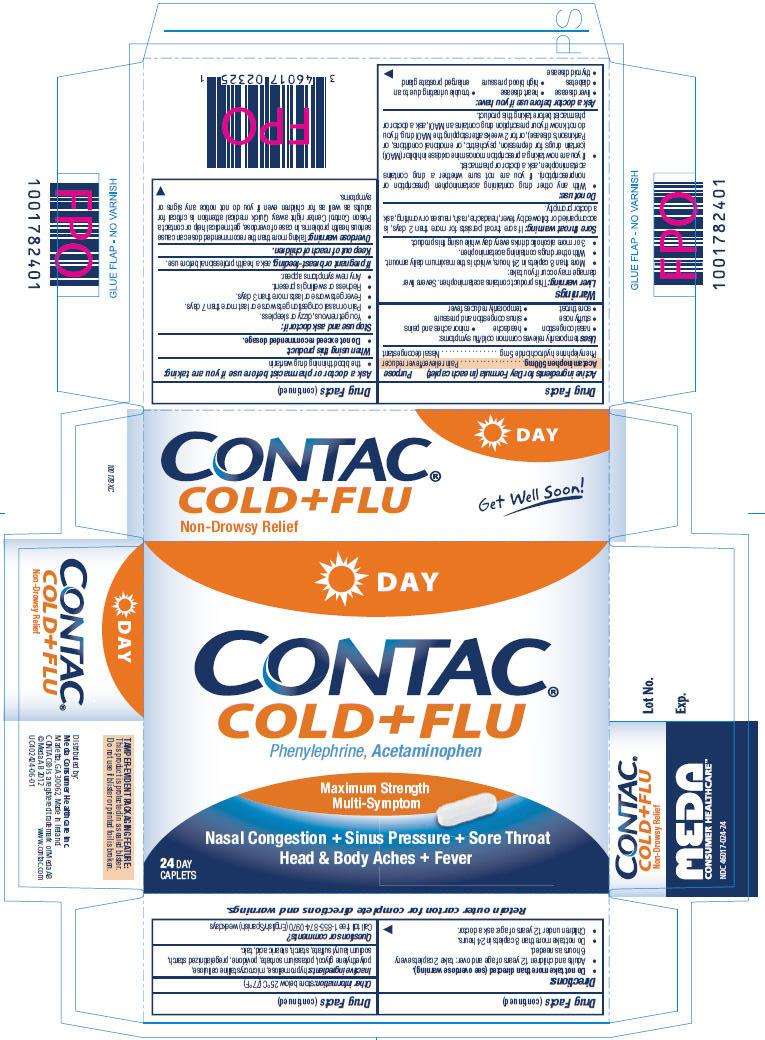
Contac Cold and Flu
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients for Day Formula (in each caplet)
- Purpose
- Contac Cold and Flu Uses
- Warnings
- Directions
- Contac Cold and Flu Other information
- Inactive ingredients
FULL PRESCRIBING INFORMATION
Active ingredients for Day Formula (in each caplet)
Acetaminophen 500 mg
Phenylephrine hydrochloride 5 mg
Purpose
Pain reliever/fever reducer
Nasal decongestant
Contac Cold and Flu Uses
- temporarily relieves common cold/flu symptoms:
- nasal congestion
- headache
- minor aches and pains
- stuffy nose
- sore throat
- sinus congestion and pressure
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- More than 8 caplets in 24 hours, which is the maximum daily amount.
- With other drugs containing acetaminophen.
- 3 or more alcoholic drinks every day while using this product.
Sore throat warning: If sore throat persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, ask a doctor promptly.
Do not use
- With any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- diabetes
- high blood pressure
- trouble urinating due to an enlarged prostate gland
- thyroid disease
Ask a doctor or pharmacist before use if you are taking
- the blood thinning drug warfarin
When using this product
- Do not exceed recommended dosage
Stop use and ask a doctor if
- You get nervous, dizzy, or sleepless
- Pain or nasal congestion gets worse or lasts more than 7 days
- Fever gets worse or lasts more than 3 days
- Redness or swelling is present
- Any new symptoms appear
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose can cause serious health problems. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- Do not take more than directed (see overdose warning)
- Adults and children 12 years of age and over: take 2 caplets every 6 hours as needed.
- Do not take more than 8 caplets in 24 hours.
- Children under 12 years of age: ask a doctor
Contac Cold and Flu Other information
- store below 25°C (77°F)
Inactive ingredients
hypromellose, microcrystalline cellulose, polyethylene glycol, potassium sorbate, povidone, pregelatinized starch, sodium lauryl sulfate, starch, stearic acid, talc.
Questions or comments? call toll-free 1-855-874-0970 (English/Spanish) weekdays
Display Panel CONTAC Cold + Flu Day Caplets: 24 count Package
DAY
CONTAC®
COLD+FLU
Phenylephrine, Acetaminophen
Maximum Strength
Multi-Symptom
Nasal Congestion + Sinus Pressure +Sore Throat
Head & Body Aches + Fever
24 Day Caplets
CONTAC®
COLD+FLU
DAY
Non-Drowsy Relief
Get Well Soon!
Retain outer carton for complete directions and warnings.
TAMPER-EVIDENT PACKAGING FEATURE: This product is protected in a sealed blister. Do not use if blister or printed foil is broken.
Distributed by:
Meda Consumer Healthcare Inc.
Marietta, GA 30062, Made in Ireland
CONTAC® is a registered trademark of Meda AB
© Meda AB 2012 www.contac.com
Contac Cold and Fluacetaminophen and phenylephrine hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||