Colgate
Colgate OPTIC WHITE
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Colgate Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
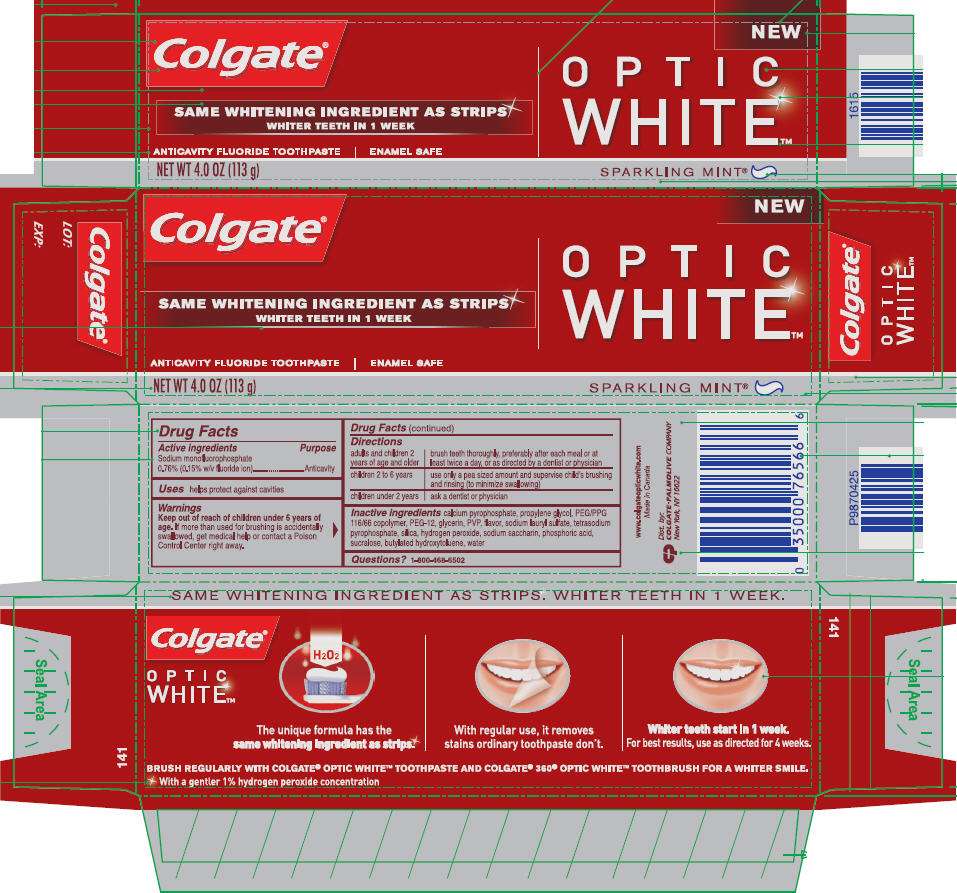
- PRINCIPAL DISPLAY PANEL - 113 g Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredients
Sodium monofluorophosphate 0.76% (0.15% w/v fluoride ion)
Purpose
Anticavity
Colgate Uses
helps protect against cavities
Warnings
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive ingredients
calcium pyrophosphate, propylene glycol, PEG/PPG 116/66 copolymer, PEG-12, glycerin, PVP, flavor, sodium lauryl sulfate, tetrasodium pyrophosphate silica, hydrogen peroxide, sodium saccharin, phosphoric acid, sucralose, butylated hydroxytoluene, water
Questions?
1-800-468-6502
PRINCIPAL DISPLAY PANEL - 113 g Carton
Colgate ®
SAME WHITENING INGREDIENT AS STRIPS
WHITER TEETH IN 1 WEEK
ANTICAVITY FLUORIDE TOOTHPASTE | ENAMEL SAFE
NET WT 4.0 OZ (113 g)
NEW
OPTIC
WHITE
™
SPARKLING MINT®
ColgateSODIUM MONOFLUOROPHOSPHATE PASTE, DENTIFRICE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||