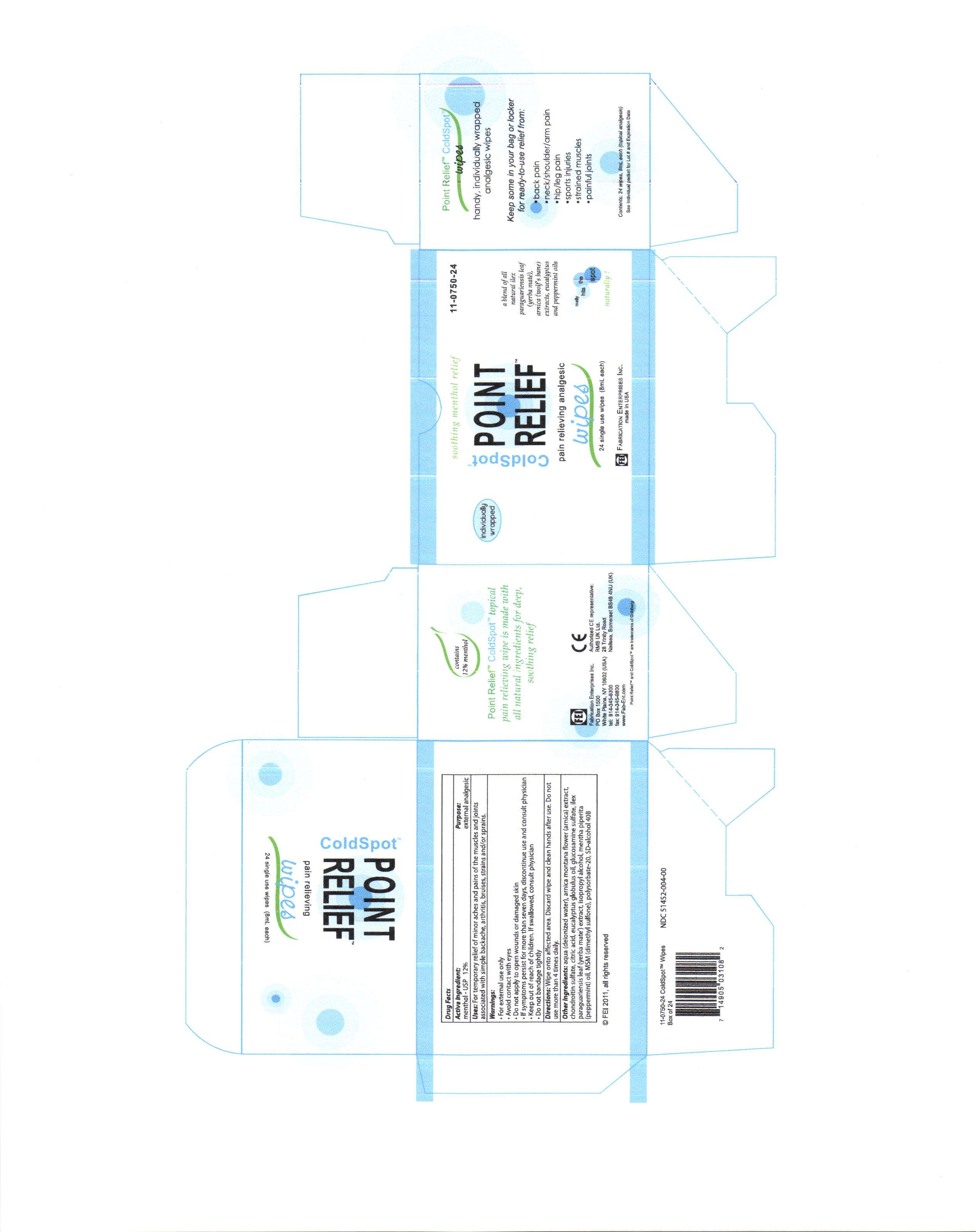
Cold Spot
Fabrication Enterprises, inc.
Pure Source
coldspot point relief
FULL PRESCRIBING INFORMATION
Active ingredient
menthol - usp 12%
aqua (deionized water), arnica montana flower (arnica) extract, chondroitin sulfate, citric acid, eucalyptus globulus oil, glucosamine sulfate,ilex paraguariensis leaf (yerba mate) extract, isopropyl alcohol, menth piperita (pepperment) oil, MSM (dimethyl sulfone) polysorbate-20, SD-alcohol 40B, triethanolamine
Keep out of reach of children. If swallowed, consult physician.
for external use only
avoid contact with eyes
do not apply to open wounds or damaged skin
if symptoms persist for more than seven days, discontinue use and consult physician
keep out of reach of children. if swallowed, consult physician
do not bandage tightly
Uses
for temporary relief of minor aches and pains of the muscles and joints associated with simple backache, arthritis, bruises, strains and/or sprains.
wipe onto affected area. discard wipe and clean hands after use. do not use more than four times per day.
Purpose
For temporary relief of minor aches and pains of the muscles and joints associated with simple backache, arthritis, bruises, strains and/or sprains.
cold spot point relief wipes
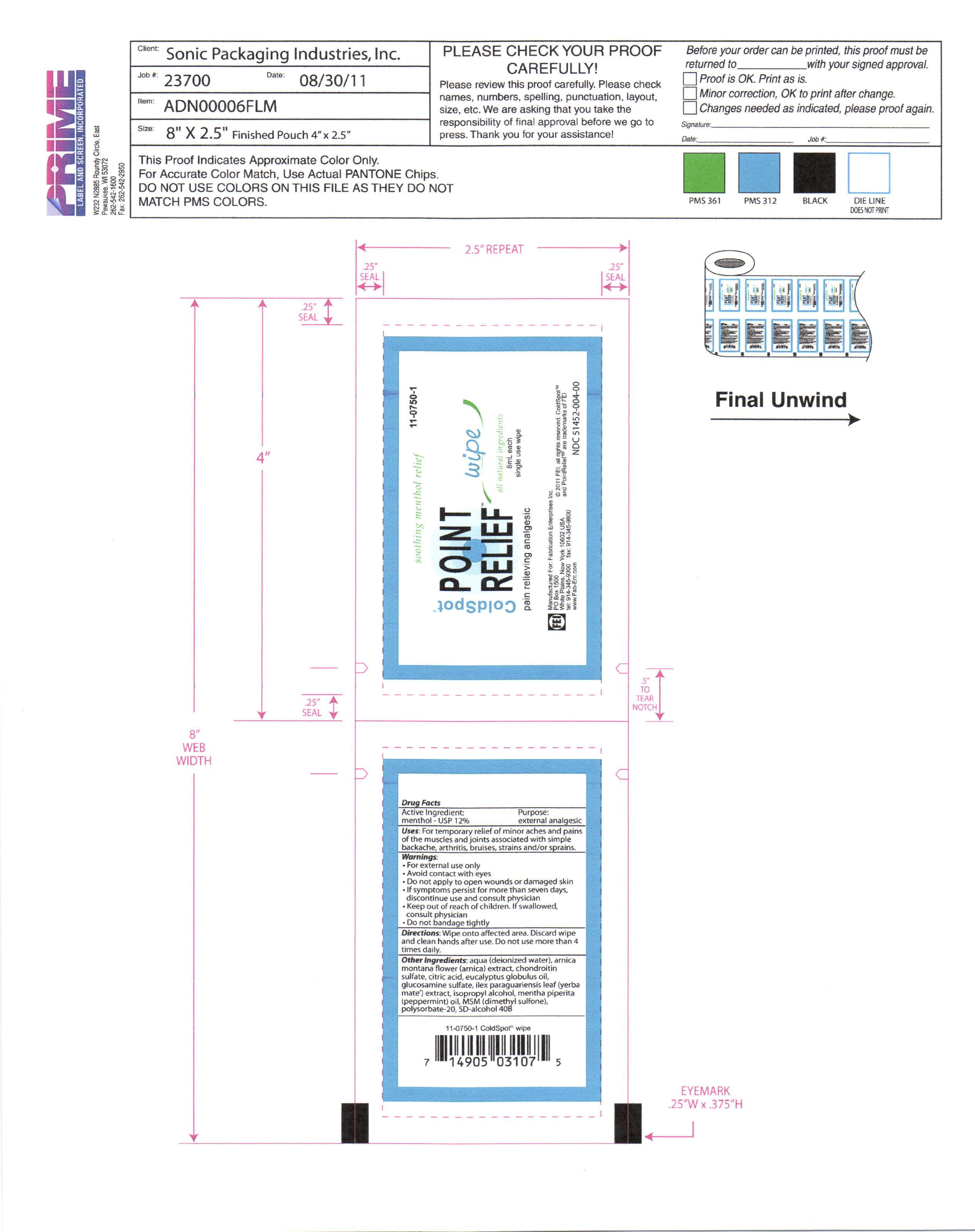
Cold SpotMENTHOL SWAB
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||