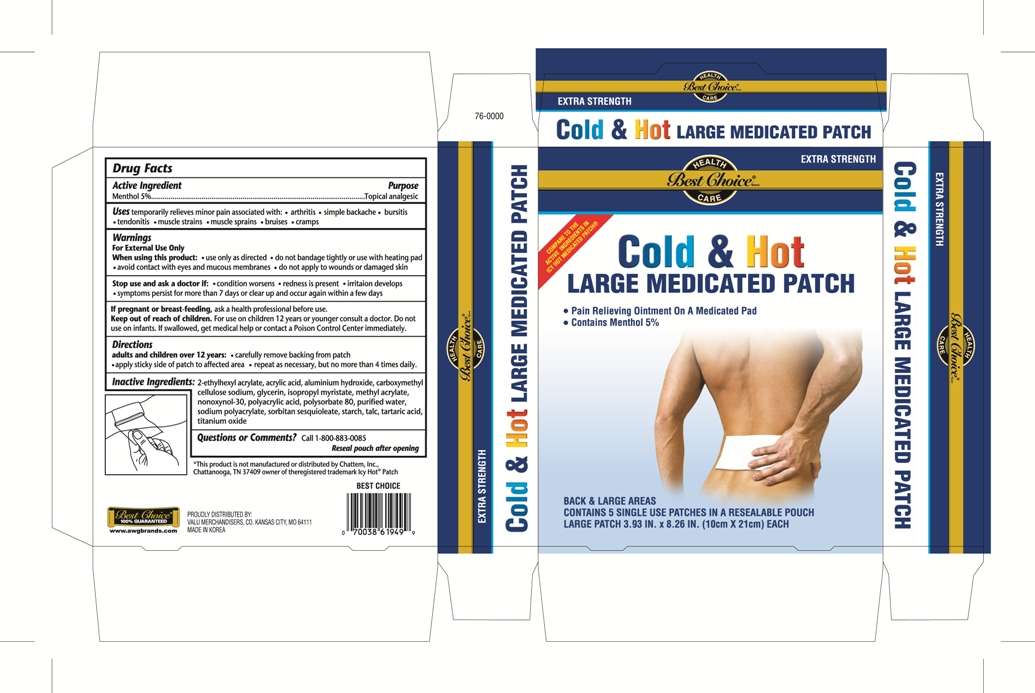
Cold and Hot Medicated Pain Relief Large
Valu Merchandisers Company, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Cold and Hot Medicated Pain Relief Large Uses
- Warnings
- Directions
- Inactive ingredients
- package label
FULL PRESCRIBING INFORMATION
Active Ingredient
Purpose
Cold and Hot Medicated Pain Relief Large Uses
- arthritis
- simple backache
- bursitis
- tendonitis
- muscle strains
- muscle sprains
- bruises
- cramps
Warnings
For external use only
When using this product
- use only as directed
- do not bandage tightly or use with heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin
Stop use and ask a doctor if
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding,
Keep out of reach of children.
For use on children 12 years or younger consult a doctor. Do not use on infants. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Adults and children over 12 years:- carefully remove backing from patch
- apply sticky side of patch to affected area
- repeat as necessary, but no more than 4 times daily
Inactive ingredients
2-ethylhexyl acrylate, acrylic acid, aluminium hydroxide, carboxymethylcellulose sodium, glycerin, isopropyl myristate, methyl acrylate, nonoxynol-30, polyacrylic acid, polysorbate 80, purified water, sodium polyacrylate, sorbitan sesquioleate, starch, talc, tartaric acid, titanium oxide
package label
Cold and Hot Medicated Pain Relief LargeMenthol PATCH
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!