Clonazepam
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLONAZEPAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- CLONAZEPAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CLONAZEPAM ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
CLONAZEPAM DESCRIPTION
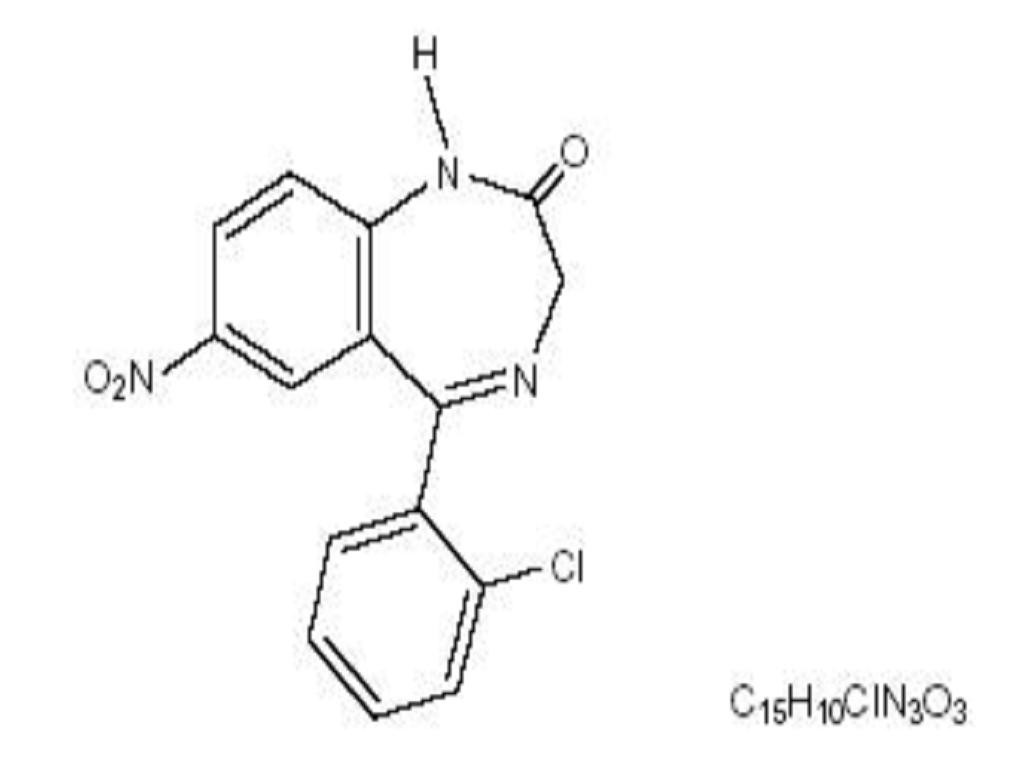
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
Clinical Trials:
INDICATIONS & USAGE
CLlNICAL PHARMACOLOGY: Clinical Trials
DOSAGE AND ADMINISTRATION
CLONAZEPAM CONTRAINDICATIONS
WARNINGS
PRECAUTIONS: Drug InteractionsInformation for PatientsPregnancy Risks:
DRUG ABUSE AND DEPENDENCE
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
WARNINGS: Pregnancy RisksPRECAUTIONS: Pregnancy
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGS: Pregnancy RisksLABOR & DELIVERY
WARNINGS: Pregnancy RisksNURSING MOTHERS
PEDIATRIC USE
INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONGERIATRIC USE
CLONAZEPAM ADVERSE REACTIONS
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY: Clinical Trials
OVERDOSAGE
DOSAGE & ADMINISTRATION
PRECAUTIONS: Geriatric Use
PRECAUTIONS: Geriatric Use
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

ClonazepamClonazepam TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!