ClomiPHENE Citrate
ClomiPHENE CITRATE TABLETS, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ClomiPHENE citrate is an orally administered, nonsteroidal, ovulatory stimulant designated chemically as 2-[p-(2-chloro-1,2-diphenylvinyl) phenoxy] triethylamine citrate (1:1). It has a molecular formula of C26H28CINO ∙C6H8O7 and a molecular weight of 598.09. It is represented structurally as:
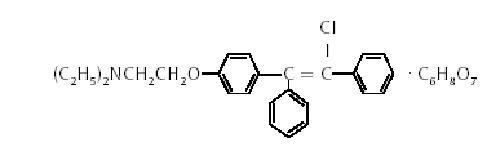
ClomiPHENE citrate is a white to pale yellow, essentially odorless, crystalline powder. It is freely soluble in methanol; soluble in ethanol; slightly soluble in acetone, water, and chloroform; and insoluble in ether.
ClomiPHENE citrate is a mixture of two geometric isomers [cis (zuclomiPHENE) and trans (enclomiPHENE)] containing between 30% and 50% of the cis-isomer.
Each white scored tablet contains 50 mg clomiPHENE citrate USP. The tablet also contains the following inactive ingredients: lactose, microcrystalline cellulose, starch, colloidal silicon dioxide, magnesium stearate and sodium starch glycolate.
Action
ClomiPHENE citrate is a drug of considerable pharmacologic potency. With careful selection and proper management of the patient, clomiPHENE citrate has been demonstrated to be a useful therapy for the anovulatory patient desiring pregnancy.
ClomiPHENE citrate is capable of interacting with estrogen-receptor-containing tissues, including the hypothalamus, pituitary, ovary, endometrium, vagina, and cervix. It may compete with estrogen for estrogen-receptor-binding sites and may delay replenishment of intracellular estrogen receptors. ClomiPHENE citrate initiates a series of endocrine events culminating in a preovulatory gonadotropin surge and subsequent follicular rupture. The first endocrine event in response to a course of clomiPHENE citrate therapy is an increase in the release of pituitary gonadotropins. This initiates steroidogenesis and folliculogenesis, resulting in growth of the ovarian follicle and an increase in the circulating level of estradiol. Following ovulation, plasma progesterone and estradiol rise and fall as they would in a normal ovulatory cycle.
Available data suggest that both the estrogenic and antiestrogenic properties of clomiPHENE may participate in the initiation of ovulation. The two clomiPHENE isomers have been found to have mixed estrogenic and antiestrogenic effects, which may vary from one species to another. Some data suggest that zuclomiPHENE has greater estrogenic activity than enclomiPHENE.
ClomiPHENE citrate has no apparent progestational, androgenic, or antiandrogenic effects and does not appear to interfere with pituitary-adrenal or pituitary-thyroid function.
Although there is no evidence of a “carryover effect” of clomiPHENE citrate, spontaneous ovulatory menses have been noted in some patients after clomiPHENE citrate therapy.
Based on early studies with 14C-labeled clomiPHENE citrate, the drug was shown to be readily absorbed orally in humans and excreted principally in the feces. Cumulative urinary and fecal excretion of the 14C averaged about 50% of the oral dose and 37% of an intravenous dose after 5 days. Mean urinary excretion was approximately 8% with fecal excretion of about 42%.
Some 14C label was still present in the feces 6 weeks after administration. Subsequent single-dose studies in normal volunteers showed that zuclomiPHENE (cis) has a longer half-life than enclomiPHENE (trans). Detectable levels of zuclomiPHENE persisted for longer than a month in these subjects. This may be suggestive of stereo-specific enterohepatic recycling or sequestering of the zuclomiPHENE. Thus, it is possible that some active drug may remain in the body during early pregnancy in women who conceive in the menstrual cycle during clomiPHENE citrate therapy.
CLINICAL STUDIES
During clinical investigations, 7578 patients received clomiPHENE citrate, some of whom had impediments to ovulation other than ovulatory dysfunction (see INDICATIONS AND USAGE). In those clinical trials, successful therapy characterized by pregnancy occurred in approximately 30% of these patients.
There were a total of 2635 pregnancies reported during the clinical trial period. Of those pregnancies, information on outcome was only available for 2369 of the cases. Table 1 summarizes the outcome of these cases.
Of the reported pregnancies, the incidence of multiple pregnancies was 7.98%: 6.9% twin, 0.5% triplet, 0.3% quadruplet, and 0.1% quintuplet. Of the 165 twin pregnancies for which sufficient information was available, the ratio of monozygotic to dizygotic twins was about 1:5. Table 1 reports the survival rate of the live multiple births.
A sextuplet birth was reported after completion of original clinical studies; none of the sextuplets survived (each weighed less than 400 g), although each appeared grossly normal.
| Outcome | Total Number of Pregnancies | Survival Rate |
| * Includes 28 ectopic pregnancies, 4 hydatidiform moles, and 1 fetus papyraceous. | ||
| † Indicates percentage of surviving infants from these pregnancies. | ||
| Pregnancy Wastage | ||
| Spontaneous Abortions | 483* | |
| Stillbirths | 24 | |
| Live Births | ||
| Single Births | 1697 | 98.16%† |
| Multiple Births | 165 | 83.25%† |
The overall survival of infants from multiple pregnancies including spontaneous abortions, stillbirths, and neonatal deaths is 73%.
ClomiPHENE citrate tablets USP is indicated for the treatment of ovulatory dysfunction in women desiring pregnancy. Impediments to achieving pregnancy must be excluded or adequately treated before beginning clomiPHENE citrate therapy. Those patients most likely to achieve success with clomiPHENE therapy include patients with polycystic ovary syndrome (see WARNINGS: Ovarian Hyperstimulation Syndrome), amenorrhea-galactorrhea syndrome, psychogenic amenorrhea, post-oral-contraceptive amenorrhea, and certain cases of secondary amenorrhea of undetermined etiology.
Properly timed coitus in relationship to ovulation is important. A basal body temperature graph or other appropriate tests may help the patient and her physician determine if ovulation occurred. Once ovulation has been established, each course of clomiPHENE citrate therapy should be started on or about the 5th day of the cycle. Long-term cyclic therapy is not recommended beyond a total of about six cycles (including three ovulatory cycles). (See DOSAGE AND ADMINISTRATION and PRECAUTIONS.)
ClomiPHENE citrate tablets USP is indicated only in patients with demonstrated ovulatory dysfunction who meet the conditions described below (see CONTRAINDICATIONS):
-
-
-
-
In addition, patients selected for clomiPHENE citrate therapy should be evaluated in regard to the following:
-
-
-
-
-
There are no adequate and well-controlled studies that demonstrate the effectiveness of clomiPHENE citrate in the treatment of male infertility. In addition, testicular tumors and gynecomastia have been reported in males using clomiPHENE. The cause and effect relationship between reports of testicular tumors and the administration of clomiPHENE citrate is not known.
Although the medical literature suggests various methods, there is no universally accepted standard regimen for combined therapy (i.e., clomiPHENE citrate in conjunction with other ovulation-inducing drugs). Similarly, there is no standard clomiPHENE citrate regimen for ovulation induction in in vitro fertilization programs to produce ova for fertilization and reintroduction. Therefore, clomiPHENE is not recommended for these uses.
Hypersensitivity
ClomiPHENE is contraindicated in patients with a known hypersensitivity or allergy to clomiPHENE citrate or to any of its ingredients.
Pregnancy
ClomiPHENE should not be administered during pregnancy. ClomiPHENE citrate may cause fetal harm in animals (see Animal Fetotoxicity). Although no causative evidence of a deleterious effect of clomiPHENE citrate therapy on the human fetus has been established, there have been reports of birth anomalies which, during clinical studies, occurred at an incidence within the range reported for the general population (see Fetal/Neonatal Anomalies and Mortality; ADVERSE REACTIONS).
To avoid inadvertent clomiPHENE citrate administration during early pregnancy, appropriate tests should be utilized during each treatment cycle to determine whether ovulation occurs. The patient should be evaluated carefully to exclude pregnancy, ovarian enlargement, or ovarian cyst formation between each treatment cycle. The next course of clomiPHENE citrate therapy should be delayed until these conditions have been excluded.
Fetal/Neonatal Anomalies and Mortality. The following fetal abnormalities have been reported subsequent to pregnancies following ovulation induction therapy with clomiPHENE citrate during clinical trials. Each of the following fetal abnormalities were reported at a rate of <1% (experiences are listed in order of decreasing frequency): Congenital heart lesions, Down syndrome, club foot, congenital gut lesions, hypospadias, microcephaly, harelip and cleft palate, congenital hip, hemangioma, undescended testicles, polydactyly, conjoined twins and teratomatous malformation, patent ductus arteriosus, amaurosis, arteriovenous fistula, inguinal hernia, umbilical hernia, syndactyly, pectus excavatum, myopathy, dermoid cyst of scalp, omphalocele, spina bifida occulta, ichthyosis, and persistent lingual frenulum. Neonatal death and fetal death/stillbirth in infants with birth defects have also been reported at a rate of <1%. The overall incidence of reported birth anomalies from pregnancies associated with maternal clomiPHENE citrate ingestion during clinical studies was within the range of that reported for the general population.
In addition, reports of birth anomalies have been received during postmarketing surveillance of clomiPHENE citrate. (see ADVERSE REACTIONS).
Animal Fetotoxicity. Oral administration of clomiPHENE citrate to pregnant rats during organogenesis at doses of 1 to 2 mg/kg/day resulted in hydramnion and weak, edematous fetuses with wavy ribs and other temporary bone changes. Doses of 8 mg/kg/day or more also caused increased resorptions and dead fetuses, dystocia, and delayed parturition, and 40 mg/kg/day resulted in increased maternal mortality. Single doses of 50 mg/kg caused fetal cataracts, while 200 mg/kg caused cleft palate.
Following injection of clomiPHENE citrate 2 mg/kg to mice and rats during pregnancy, the offspring exhibited metaplastic changes of the reproductive tract. Newborn mice and rats injected during the first few days of life also developed metaplastic changes in uterine and vaginal mucosa, as well as premature vaginal opening and anovulatory ovaries. These findings are similar to the abnormal reproductive behavior and sterility described with other estrogens and antiestrogens.
In rabbits, some temporary bone alterations were seen in fetuses from dams given oral doses of 20 or 40 mg/kg/day during pregnancy, but not following 8 mg/kg/day. No permanent malformations were observed in those studies. Also, rhesus monkeys given oral doses of 1.5 to 4.5 mg/kg/day for various periods during pregnancy did not have any abnormal offspring.
Liver Disease. ClomiPHENE citrate therapy is contraindicated in patients with liver disease or a history of liver dysfunction (see also INDICATIONS AND USAGE and ADVERSE REACTIONS).
Abnormal Uterine Bleeding. ClomiPHENE citrate is contraindicated in patients with abnormal uterine bleeding of undetermined origin (see INDICATIONS AND USAGE).
Ovarian Cysts. ClomiPHENE citrate is contraindicated in patients with ovarian cysts or enlargement not due to polycystic ovarian syndrome (see INDICATIONS AND USAGE and WARNINGS).
Other. ClomiPHENE citrate is contraindicated in patients with uncontrolled thyroid or adrenal dysfunction or in the presence of an organic intracranial lesion such as pituitary tumor (see INDICATIONS and USAGE).
Visual Symptoms
Patients should be advised that blurring or other visual symptoms such as spots or flashes (scintillating scotomata) may occasionally occur during therapy with clomiPHENE citrate. These visual symptoms increase in incidence with increasing total dose or therapy duration and generally disappear within a few days or weeks after clomiPHENE citrate therapy is discontinued. However, prolonged visual disturbances have been reported after clomiPHENE citrate therapy has been discontinued and these disturbances may be irreversible. Patients should be warned that these visual symptoms may render such activities as driving a car or operating machinery more hazardous than usual, particularly under conditions of variable lighting.
These visual symptoms appear to be due to intensification and prolongation of afterimages. Symptoms often first appear or are accentuated with exposure to a brightly lit environment. While measured visual acuity usually has not been affected, a study patient taking 200 mg clomiPHENE citrate daily developed visual blurring on the 7th day of treatment, which progressed to severe diminution of visual acuity by the 10th day. No other abnormality was found, and the visual acuity returned to normal on the 3rd day after treatment was stopped.
Ophthalmologically definable scotomata and retinal cell function (electroretinographic) changes have also been reported. A patient treated during clinical studies developed phosphenes and scotomata during prolonged clomiPHENE citrate administration, which disappeared by the 32nd day after stopping therapy.
Postmarketing surveillance of adverse events has also revealed other visual signs and symptoms during clomiPHENE citrate therapy (see ADVERSE REACTIONS).
While the etiology of these visual symptoms is not yet understood, patients with any visual symptoms should discontinue treatment and have a complete ophthalmological evaluation carried out promptly.
Ovarian Hyperstimulation Syndrome
The ovarian hyperstimulation syndrome (OHSS) has been reported to occur in patients receiving clomiPHENE citrate therapy for ovulation induction. In some cases, OHSS occurred following cyclic use of clomiPHENE citrate therapy or when clomiPHENE citrate was used in combination with gonadotropins. Transient liver function test abnormalities suggestive of hepatic dysfunction, which may be accompanied by morphologic changes on liver biopsy, have been reported in association with ovarian hyperstimulation syndrome (OHSS).
OHSS is a medical event distinct from uncomplicated ovarian enlargement. It may progress rapidly (within 24 hours to several days) to become a serious medical event. The clinical signs of this syndrome in severe cases can include gross ovarian enlargement, gastrointestinal symptoms, ascites, dyspnea, oliguria, and pleural effusion. In addition, the following symptoms have been reported in association with this syndrome: pericardial effusion, anasarca, hydrothorax, acute abdomen, hypotension, renal failure, pulmonary edema, intraperitoneal and ovarian hemorrhage, deep venous thrombosis, torsion of the ovary, and acute respiratory distress. The early warning signs of OHSS are abdominal pain and distention, nausea, vomiting, diarrhea, and weight gain. Elevated urinary steroid levels, varying degrees of electrolyte imbalance, hypovolemia, hemoconcentration, and hypoproteinemia may occur. Death due to hypovolemic shock, hemoconcentration, or thromboembolism has occurred. Due to fragility of enlarged ovaries in severe cases, abdominal and pelvic examination should be performed very cautiously. If conception results, rapid progression to the severe form of the syndrome may occur.
To minimize the hazard associated with occasional abnormal ovarian enlargement associated with clomiPHENE citrate therapy, the lowest dose consistent with expected clinical results should be used. Maximal enlargement of the ovary, whether physiologic or abnormal, may not occur until several days after discontinuation of the recommended dose of clomiPHENE citrate. Some patients with polycystic ovary syndrome who are unusually sensitive to gonadotropin may have an exaggerated response to usual doses of clomiPHENE citrate. Therefore, patients with polycystic ovary syndrome should be started on the lowest recommended dose and shortest treatment duration for the first course of therapy (see DOSAGE AND ADMINISTRATION).
If enlargement of the ovary occurs, additional clomiPHENE citrate therapy should not be given until the ovaries have returned to pretreatment size, and the dosage or duration of the next course should be reduced. Ovarian enlargement and cyst formation associated with clomiPHENE citrate therapy usually regress spontaneously within a few days or weeks after discontinuing treatment. The potential benefit of subsequent clomiPHENE citrate therapy in these cases should exceed the risk. Unless surgical indication for laparotomy exists, such cystic enlargement should always be managed conservatively.
A causal relationship between ovarian hyperstimulation and ovarian cancer has not been determined. However, because a correlation between ovarian cancer and nulliparity, infertility, and age has been suggested, if ovarian cysts do not regress spontaneously, a thorough evaluation should be performed to rule out the presence of ovarian neoplasia.
General
Careful attention should be given to the selection of candidates for clomiPHENE citrate therapy. Pelvic examination is necessary prior to clomiPHENE citrate treatment and before each subsequent course (see CONTRAINDICATIONS and WARNINGS).
Information for Patients
The purpose and risks of clomiPHENE citrate therapy should be presented to the patient before starting treatment. It should be emphasized that the goal of clomiPHENE citrate therapy is ovulation for subsequent pregnancy. The physician should counsel the patient with special regard to the following potential risks:
Visual Symptoms: Advise that blurring or other visual symptoms occasionally may occur during or shortly after clomiPHENE citrate therapy. It should be made clear that, in some instances, visual disturbances may be prolonged and, possibly, irreversible. Warn that visual symptoms may render such activities as driving a car or operating machinery more hazardous than usual, particularly under conditions of variable lighting (see WARNINGS).
The patient should be instructed to inform the physician whenever any unusual visual symptoms occur. If the patient has any visual symptoms, treatment should be discontinued and complete ophthalmologic evaluation performed.
Abdominal/Pelvic Pain or Distention: Ovarian enlargement may occur during or shortly after therapy with clomiPHENE citrate. To minimize the risks associated with ovarian enlargement, the patient should be instructed to inform the physician of any abdominal or pelvic pain, weight gain, discomfort, or distention after taking clomiPHENE citrate (see WARNINGS).
Multiple Pregnancy: Inform the patient that there is an increased chance of multiple pregnancy, including bilateral tubal pregnancy and coexisting tubal and intrauterine pregnancy, when conception occurs in relation to clomiPHENE citrate therapy. The potential complications and hazards of multiple pregnancy should be explained.
Pregnancy Wastage and Birth Anomalies: The physician should explain the assumed risk of any pregnancy, whether ovulation is induced with the aid of clomiPHENE citrate or occurs naturally. The patient should be informed of the greater risks associated with certain characteristics or conditions of any pregnant woman, eg, age of female and male partner, history of spontaneous abortions, Rh genotype, abnormal menstrual history, infertility history, organic heart disease, diabetes, exposure to infectious agents such as rubella, familial history of birth anomaly, that may be pertinent to the patient for whom clomiPHENE citrate is being considered. Based upon the evaluation of the patient, genetic counseling may be indicated.
The overall incidence of reported birth anomalies from pregnancies associated with maternal clomiPHENE citrate ingestion during the investigational studies was within the range of that reported in published references for the general population. (See CONTRAINDICATIONS: Pregnancy.)
During clinical investigation, the experience from patients with known pregnancy outcome (Table 1) show a spontaneous abortion rate of 20.4% and stillbirth rate of 1.0%. (See CLINICAL PHARMACOLOGY)
Drug Interactions
Drug interactions with clomiPHENE citrate have not been documented.
Long-term toxicity studies in animals have not been performed to evaluate the carcinogenic or mutagenic potential of clomiPHENE citrate.
Oral administration of clomiPHENE citrate to male rats at doses of 0.3 or 1 mg/kg/day caused decreased fertility, while higher doses caused temporary infertility. Oral doses of 0.1 mg/kg/day in female rats temporarily interrupted the normal cyclic vaginal smear pattern and prevented conception. Doses of 0.3 mg/kg/day slightly reduced the number of ovulated ova and corpora lutea, while 3 mg/kg/day inhibited ovulation.
Pregnancy
Pregnancy Category X. (See CONTRAINDICATIONS.)
Nursing Mothers
It is not known whether clomiPHENE citrate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if clomiPHENE citrate is administered to a nursing woman. In some patients, clomiPHENE citrate may reduce lactation.
Ovarian Cancer
Prolonged use of clomiPHENE citrate tablets USP may increase the risk of a borderline or invasive ovarian tumor (see ADVERSE REACTIONS).
Clinical Trial Adverse Events
ClomiPHENE citrate, at recommended dosages, is generally well tolerated. Adverse reactions usually have been mild and transient and most have disappeared promptly after treatment has been discontinued. Adverse experiences reported in patients treated with clomiPHENE citrate during clinical studies are shown in Table 2.
| Adverse Event | % | |
| *Includes 498 patients whose reports may have been duplicated in the event totals and could not be distinguished as such. Also, excludes 47 patients who did not report symptom data. | ||
| Ovarian Enlargement | 13.6 | |
| Vasomotor Flushes | 10.4 | |
| Abdominal-Pelvic Discomfort/Distention/Bloating | 5.5 | |
| Nausea and Vomiting | 2.2 | |
| Breast Discomfort | 2.1 | |
| Visual Symptoms | 1.5 | |
| Blurred vision, lights, floaters, waves, unspecified visual complaints, photophobia, diplopia, scotomata, phosphenes | ||
| Headache | 1.3 | |
| Abnormal Uterine Bleeding | 1.3 | |
| Intermenstrual spotting, menorrhagia | ||
The following adverse events have been reported in fewer than 1% of patients in clinical trials: Acute abdomen, appetite increase, constipation, dermatitis or rash, depression, diarrhea, dizziness, fatigue, hair loss/dry hair, increased urinary frequency/volume, insomnia, light-headedness, nervous tension, vaginal dryness, vertigo, weight gain/loss.
Patients on prolonged clomiPHENE citrate therapy may show elevated serum levels of desmosterol. This is most likely due to a direct interference with cholesterol synthesis. However, the serum sterols in patients receiving the recommended dose of clomiPHENE citrate are not significantly altered. Ovarian cancer has been infrequently reported in patients who have received fertility drugs. Infertility is a primary risk factor for ovarian cancer; however, epidemiology data suggest that prolonged use of clomiPHENE may increase the risk of a borderline or invasive ovarian tumor.
Postmarketing Adverse Events
The following adverse experiences were reported spontaneously with clomiPHENE citrate. The cause and effect relationship of the listed events to the administration of clomiPHENE citrate is not known.
Dermatologic: Acne, allergic reaction, erythema, erythema multiforme, erythema nodosum, hypertrichosis, pruritus, urticaria.
Central Nervous System: Migraine headache, paresthesia, seizure, stroke, syncope
Psychiatric: Anxiety, irritability, mood changes, psychosis
Visual Disorders: Abnormal accommodation, cataract, eye pain, macular edema, optic neuritis, photopsia, posterior vitreous detachment, retinal hemorrhage, retinal thrombosis, retinal vascular spasm, temporary loss of vision
Cardiovascular: Arrhythmia, chest pain, edema, hypertension, palpitation, phlebitis, pulmonary embolism, shortness of breath, tachycardia, thrombophlebitis
Musculoskeletal: Arthralgia, back pain, myalgia
Hepatic: Transaminases increased, hepatitis
Neoplasms: Liver (hepatic hemangiosarcoma, liver cell adenoma, hepatocellular carcinoma); breast (fibrocystic disease, breast carcinoma); endometrium (endometrial carcinoma); nervous system (astrocytoma, pituitary tumor, prolactinoma, neurofibromatosis, glioblastoma multiforme, brain abscess); ovary (luteoma of pregnancy, dermoid cyst of the ovary, ovarian carcinoma); trophoblastic (hydatiform mole, choriocarcinoma); miscellaneous (melanoma, myeloma, perianal cysts, renal cell carcinoma, Hodgkin’s lymphoma, tongue carcinoma, bladder carcinoma): and neoplasms of offspring (neuroectodermal tumor, thyroid tumor, hepatoblastoma, lymphocytic leukemia).
Genitourinary: Endometriosis, ovarian cyst (ovarian enlargement or cysts could, as such, be complicated by adnexal torsion), ovarian hemorrhage, tubal pregnancy, uterine hemorrhage.
Body as a Whole: Fever, tinnitus, weakness
Other: Leukocytosis, thyroid disorder
Fetal/Neonatal Anomalies: The following fetal abnormalities have also been reported during postmarketing surveillance: delayed development; abnormal bone development including skeletal malformations of the skull, face, nasal passages, jaw, hand, limb (ectromelia including amelia, hemimelia, and phocomelia), foot, and joints; tissue malformations including imperforate anus, tracheoesophageal fistula, diaphragmatic hernia, renal agenesis and dysgenesis, and malformations of the eye and lens (cataract), ear, lung, heart (ventricular septal defect and tetralogy of Fallot), and genitalia; as well as dwarfism, deafness, mental retardation, chromosomal disorders, and neural tube defects (including anencephaly).
DRUG ABUSE AND DEPENDENCE
Tolerance, abuse, or dependence with clomiPHENE citrate has not been reported.
Signs and Symptoms
Toxic effects accompanying acute overdosage of clomiPHENE citrate have not been reported. Signs and symptoms of overdosage as a result of the use of more than the recommended dose during clomiPHENE citrate therapy include nausea, vomiting, vasomotor flushes, visual blurring, spots or flashes, scotomata, ovarian enlargement with pelvic or abdominal pain. (See CONTRAINDICATIONS: Ovarian Cyst.)
Oral LD 50 : The acute oral LD50 of clomiPHENE citrate is 1700 mg/kg in mice and 5750 mg/kg in rats. The toxic dose in humans is not known.
Dialysis: It is not known if clomiPHENE citrate is dialyzable.
Treatment
In the event of overdose, appropriate supportive measures should be employed in addition to gastrointestinal decontamination.
General Considerations
The workup and treatment of candidates for clomiPHENE citrate therapy should be supervised by physicians experienced in management of gynecologic or endocrine disorders. Patients should be chosen for therapy with clomiPHENE citrate only after careful diagnostic evaluation (see INDICATIONS AND USAGE). The plan of therapy should be outlined in advance. Impediments to achieving the goal of therapy must be excluded or adequately treated before beginning clomiPHENE citrate. The therapeutic objective should be balanced with potential risks and discussed with the patient and others involved in the achievement of a pregnancy.
Ovulation most often occurs from 5 to 10 days after a course of clomiPHENE citrate. Coitus should be timed to coincide with the expected time of ovulation. Appropriate tests to determine ovulation may be useful during this time.
Recommended Dosage
Treatment of the selected patient should begin with a low dose, 50 mg daily (1 tablet) for 5 days. The dose should be increased only in those patients who do not ovulate in response to cyclic 50 mg clomiPHENE citrate. A low dosage or duration of treatment course is particularly recommended if unusual sensitivity to pituitary gonadotropin is suspected, such as in patients with polycystic ovary syndrome (see WARNINGS: Ovarian Hyperstimulation Syndrome).
The patient should be evaluated carefully to exclude pregnancy, ovarian enlargement, or ovarian cyst formation between each treatment cycle.
If progestin-induced bleeding is planned, or if spontaneous uterine bleeding occurs prior to therapy, the regimen of 50 mg daily for 5 days should be started on or about the 5th day of the cycle. Therapy may be started at any time in the patient who has had no recent uterine bleeding. When ovulation occurs at this dosage, there is no advantage to increasing the dose in subsequent cycles of treatment.
If ovulation does not appear to occur after the first course of therapy, a second course of 100 mg daily (two 50 mg tablets given as a single daily dose) for 5 days should be given. This course may be started as early as 30 days after the previous one after precautions are taken to exclude the presence of pregnancy. Increasing the dosage or duration of therapy beyond 100 mg/day for 5 days is not recommended.
The majority of patients who are going to ovulate will do so after the first course of therapy. If ovulation does not occur after three courses of therapy, further treatment with clomiPHENE citrate is not recommended and the patient should be reevaluated. If three ovulatory responses occur, but pregnancy has not been achieved, further treatment is not recommended. If menses does not occur after an ovulatory response, the patient should be reevaluated. Long-term cyclic therapy is not recommended beyond a total of about six cycles (see PRECAUTIONS).
ClomiPHENE Citrate Tablets, USP are available as 50 mg scored white tablets in the following package combinations:
• 1 carton 10 tablets, NDC 0093-0041-03
Each carton contains 2 strips of 5 tablets each.
• 1 carton 30 tablets, NDC 0093-0041-65
Each carton contains 3 strips of 10 tablets, each
in a 2 x 5 arrangement.
Store tablets at controlled room temperature 59-86°F (15-30°C) [See USP]. Protect from heat, light, excessive humidity, and store in closed containers.
Rx Only
Manufactured In Israel By:
TEVA PHARMACEUTICAL INDUSTRIES LTD.
Jerusalem, 91010, Israel
Manufactured For:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
June 2010
PRINCIPAL DISPLAY PANEL

ClomiPHENE Citrate Tablets USP 50 mg 30s Carton Text
NDC 0093-0041-65
6505-00-181-7678
ClomiPHENE CITRATE Tablets, USP
50 mg
USUAL DOSE: 1 tablet for 5 days. See enclosed prescribing information
before using.
Rx only
30 TABLETS
TEVA
ClomiPHENE CitrateClomiPHENE citrate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||