Clobetasol Propionate
FULL PRESCRIBING INFORMATION: CONTENTS*
- Clobetasol Propionate Foam, 0.05%
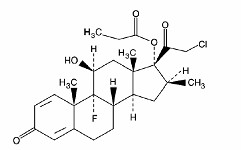
- CLOBETASOL PROPIONATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- Pharmacokinetics
- CLINICAL STUDIES
- CLOBETASOL PROPIONATE INDICATIONS AND USAGE
- CLOBETASOL PROPIONATE CONTRAINDICATIONS
- PRECAUTIONS
- Information for Patients
- Laboratory Tests
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Pregnancy
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- CLOBETASOL PROPIONATE ADVERSE REACTIONS
- OVERDOSAGE
- CLOBETASOL PROPIONATE DOSAGE AND ADMINISTRATION
- Instructions for applying clobetasol propionate foam, 0.05%
- HOW SUPPLIED
- WARNING
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Clobetasol Propionate Foam, 0.05%
For Dermatologic Use Only
Not for Ophthalmic Use
CLOBETASOL PROPIONATE DESCRIPTION
25325
CLINICAL PHARMACOLOGY
22
Pharmacokinetics
CLINICAL STUDIES
|
|
Clobetasol Propionate Foam, 0.05% n (%) |
Vehicle Foam n (%) |
| Total number of subjects | 139 | 140 |
| Subjects with Treatment Success* | 39 (28) | 4 (3) |
| Physician's Static Global Assessment - Clear or Almost Clear at Endpoint | 94 (68) | 30 (21) |
| Scaling - Clear or Almost Clear at Endpoint | 101 (73) | 42 (30) |
| Erythema - Clear or Almost Clear at Endpoint | 88 (63) | 35 (25) |
| Plaque Thickness - Clear at Endpoint | 44 (32) | 5 (4) |
CLOBETASOL PROPIONATE INDICATIONS AND USAGE
CLOBETASOL PROPIONATE CONTRAINDICATIONS
PRECAUTIONS
General: Clobetasol propionate is a super-potent topical corticosteroid that has been shown to suppress the ad renaIs at 7.0 g of clobetasol propionate foam, 0.05% per day. Lesser amounts of clobetasol propionate foam, 0.05% were not studied.
Information for Patients
- This medication is to be used as directed by the physician and should not be used longer than the prescribed time period. It is for external use only. Avoid contact with the eyes.
- This medication should not be used for any disorder other than that for which it was prescribed.
- The treated area should not be bandaged or otherwise covered or wrapped so as to be occlusive unless directed by the physician.
- Patients should report to their physician any signs of local adverse reactions.
Laboratory Tests
ACTH stimulation test
A.M. plasma cortisol test
Urinary free cortisol test
Carcinogenesis, Mutagenesis, Impairment of Fertility
Clobetasol propionate was non-mutagenic in three different test systems: the Ames test, the Saccharomyces cerevisiae gene conversion assay, and the E. coli B WP2 fluctuation test.
Studies in the rat following subcutaneous administration of clobetasol propionate at dosage levels up to 0.05 mg/kg per day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose.
Pregnancy
Pregnancy category C
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application to laboratory animals.
Clobetasol propionate has not been tested for teratogenicity by the topical route; however, it is absorbed percutaneously, and when administered subcutaneously, it was a significant teratogen in both the rabbit and the mouse. Clobetasol propionate has greater teratogenic potential than steroids that are less potent.
Teratogenicity studies in mice using the subcutaneous route resulted in fetotoxicity at the highest dose tested (1 mg/kg) and teratogenicity at all dose levels tested down to 0.03 mg/kg. These doses are approximately 1.4 and 0.04 times, respectively, the human topical dose of clobetasol propionate based on body surface area comparisons. Abnormalities seen included cleft palate and skeletal abnormalities.
In rabbits, clobetasol propionate was teratogenic at doses of 0.003 and 0.01 mg/kg. These doses are approximately 0.02 and 0.05 times, respectively, the human topical dose of clobetasol propionate based on body surface area comparisons. Abnormalities seen included cleft palate, cranioschisis, and other skeletal abnormalities.
There are no adequate and well-controlled studies of the teratogenic potential of clobetasol propionate in pregnant women. Clobetasol propionate foam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
Pediatric Use
Safety and effectiveness of clobetasol propionate foam in pediatric patients have not been established; therefore, use in children under 12 years of age is not recommended. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of adrenal suppression and Cushing’s syndrome when they are treated with topical corticosteroids. Pediatric patients are therefore at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
Adrenal suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Geriatric Use
CLOBETASOL PROPIONATE ADVERSE REACTIONS
| Dermatosis | Clobetasol Propionate Foam, 0.05% |
| Psoriasis | 1 of 9 |
| Atopic Dermatatitis* | 4 of 4 |
OVERDOSAGE
CLOBETASOL PROPIONATE DOSAGE AND ADMINISTRATION
Note: For proper dispensing of foam, hold the can upside down and depress the actuator.
Clobetasol propionate foam, 0.05% should be applied to the affected area twice daily, once in the morning and once at night. Invert the can and dispense a small amount of clobetasol propionate foam, 0.05% (up to a maximum of a golf-ball-size dollop or one and a half capfuls) into the cap of the can, onto a saucer or other cool surface, or to the lesion, taking care to avoid contact with the eyes. Dispensing directly onto hands is not recommended (unless the hands are the affected area), as the foam will begin to melt immediately upon contact with warm skin. When applying clobetasol propionate foam, 0.05% to a hair-bearing area, move the hair away from the affected area so that the foam can be applied to each affected area. Pick up small amounts with fingertips and gently massage into affected area until the foam disappears. Repeat until entire affected area is treated.
Apply the smallest amount possible that sufficiently covers the affected area(s). No more than one and a half capfuls of foam should be used at each application. Do not apply to face or intertriginous areas.
Clobetasol propionate foam, 0.05% is a super-high-potency topical corticosteroid; therefore, treatment should be limited to 2 consecutive weeks and amounts greater than 50 g/week should not be used. Use in pediatric patients under 12 years of age is not recommended.
Unless directed by a physician, clobetasol propionate foam, 0.05% should not be used with occlusive dressings.
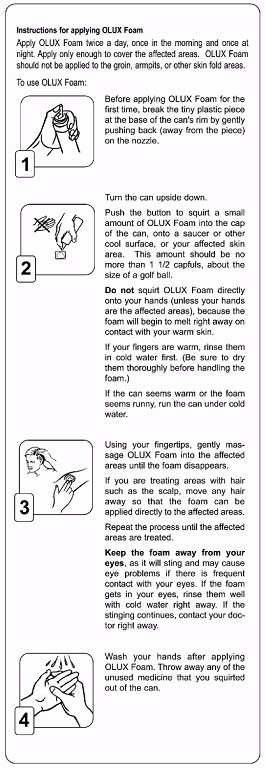
Instructions for applying clobetasol propionate foam, 0.05%
- Before applying clobetasol propionate foam ,0.05% for the first time, break the tiny plastic piece at the base of the can's rim by gently pushing back (away from the piece)on the nozzle.
- Turn the can upside down. Push the button to squirt a small amount of clobetasol propionate foam, 0.05% into the cap of the can, onto a saucer or other cool surface,or your affected skin area. This amount should be no more than 1 1/2 capfuls, about the size of a golf ball. Do not squirt clobetasol propionate foam, 0.05% directly onto your hands (unless your hands are the affected areas), because the foam will begin to melt right away on contact with your warm skin. If your fingers are warm, rinse them in cold water first. (Be sure to dry them thoroughly before handling the foam.) If the can seems warm or the foam seems runny, run the can under cold water.
- Using your fingertips, gently massage clobetasol propionate foam, 0.05% into the affected areas until the foam disappears. If you are treating areas with hair such as the scalp, move any hair away so that the foam can be applied directly to the affected areas.Repeat the process until the affected areas are treated. Keep the foam away from your eyes, as it will sting and may cause eye problems if there is frequent contact with your eyes. If the foam gets in your eyes, rinse them well with cold water right away. If the stinging continues, contact your doctor right away.
- Wash your hands after applying clobetasol propionate foam, 0.05%. Throwaway any of the unused medicine that you squirted out of the can.
HOW SUPPLIED
oo
WARNING
ooPrincipal Display Panel



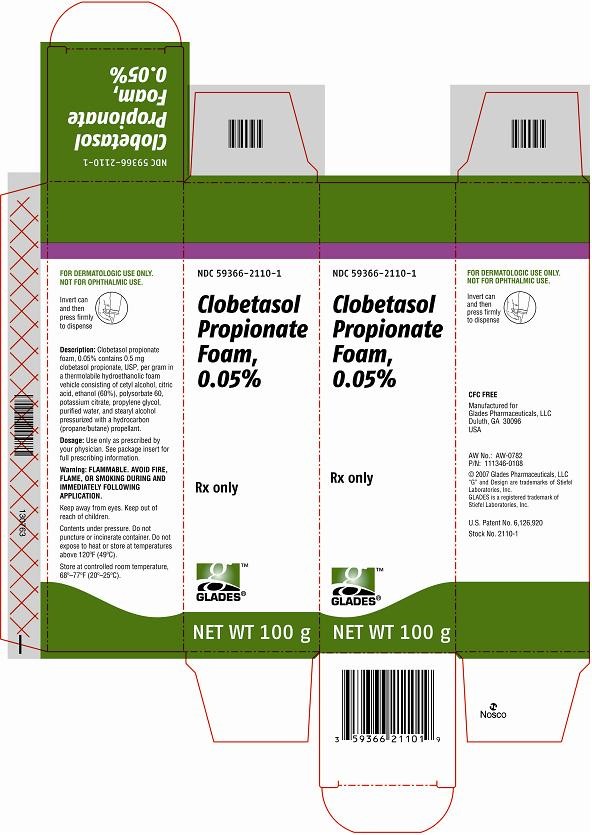
Clobetasol PropionateClobetasol Propionate AEROSOL, FOAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||