Clenia
Upsher-Smith Laboratories, Inc
Clenia (sodium sulfacetamide 10% and sulfur 5%) Foaming Wash and Emollient Cream
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLENIA DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS
- CLENIA CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CLENIA ADVERSE REACTIONS
- CLENIA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 28 g tube label
- PRINCIPAL DISPLAY PANEL - 28 g tube carton
- PRINCIPAL DISPLAY PANEL - 3 g sample tube label
- PRINCIPAL DISPLAY PANEL - 3 g sample tube carton
- PRINCIPAL DISPLAY PANEL - 170 g bottle label
- PRINCIPAL DISPLAY PANEL - 170 g bottle carton
- PRINCIPAL DISPLAY PANEL - 340 g bottle label
- PRINCIPAL DISPLAY PANEL - 340 g bottle carton
- PRINCIPAL DISPLAY PANEL - 5 g sample bottle label
- PRINCIPAL DISPLAY PANEL - 5 g sample bottle carton
FULL PRESCRIBING INFORMATION
Rx only
CLENIA DESCRIPTION
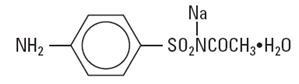
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically, sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate.
The structural formula is:
Each gram of CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Foaming Wash contains 100 mg of Sodium Sulfacetamide and 50 mg of Sulfur in a base containing Butylated Hydroxytoluene, NF, Disodium EDTA, USP, Disodium Oleamido MEA Sulfosuccinate, Fragrance, Glyceryl Stearate (and) PEG-100 Stearate, Magnesium Aluminum Silicate, NF, Methylparaben, NF, Mica, PEG-55 Propylene Glycol Oleate, Propylene Glycol, USP, Propylparaben, NF, Purified Water, USP, Silicon Dioxide, Sodium Cocyl Isethionate, Sodium Methyl Oleytaurate, Sodium Thiosulfate, USP, Stearic Acid, NF, Titanium Dioxide, Xanthan Gum, NF.
Each gram of CLENIA® (sodium sulfacetamide 10% and sulfur 5%0 Emollient Cream contains 100 mg of Sodium Sulfacetamide and 50 mg of Sulfur in a base containing Allantoin, Disodium EDTA, USP, Emulsifying Wax, NF, Fragrance, Glyceryl Monostearate, Isopropyl Myristate, NF, Methylparaben, NF, PEG-8 Stearate, Propylene Glycol, USP, Propylparaben, NF, Purified Water, USP, Sodium Thiosulfate, USP, Stearic Acid, NF, Xanthan Gum, NF.
CLINICAL PHARMACOLOGY
Sodium sulfacetamide exhibits antibacterial activity. It is believed to block bacterial growth by acting as a competitive antagonist of para-aminobenzoic acid (PABA). While absorption through intact skin has not been determined for sodium sulfacetamide, it is estimated that 1% of topically applied sulfur is absorbed. Although the exact mode of the keratolytic activity of sulfur is unknown, it is reported to result from the interaction of sulfur with the cysteine content of keratinocytes. In combination with sulfacetamide, sulfur has been reported to inhibit P. acnes, thereby reducing the associated inflammation.
INDICATIONS
CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Foaming Wash and CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Emollient Cream are indicated in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
CLENIA CONTRAINDICATIONS
CLENIA® Foaming Wash and CLENIA® Emollient Cream are contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of these preparations. CLENIA® Foaming Wash and CLENIA® Emollient Cream are not to be used by patients with kidney disease.
WARNINGS
Although rare, hypersensitivity reactions to products containing sodium sulfacetamide may occur, including Stevens-Johnson syndrome and exfoliative dermatitis. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura, hemorrhagica, drug fever, jaundice and contact dermatitis indicate hypersensivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.
FOR EXTERNAL USE ONLY
Keep away from eyes. Keep out of reach of children.
PRECAUTIONS
General
Although the object of this therapy is to achieve desquamation without irritation, this product poses a low potential for irritation, which may include reddening and scaling of the epidermis. Uncommon adverse reactions such as dryness, erythema, itching and edema may occur. Therefore, patients should be monitored for possible local irritation or sensitization. Use of the product should be discontinued if excessive irritation develops.
Information for Patients
Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. If excessive irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Foaming Wash or CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Emollient Cream. It is not known whether CLENIA® Foaming Wash and CLENIA® Emollient Cream can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. CLENIA® Foaming Wash and CLENIA® Emollient Cream should be used during pregnancy only if the potential benefit outweighs the potential risk.
Nursing Mothers
It is not known whether sodium sulfacetamide is excreted in human milk following topical use of CLENIA® Foaming Wash and CLENIA® Emollient Cream. However, small amounts of orally administered sulfonamides have been detected in human milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when CLENIA® Foaming Wash and CLENIA® Emollient Cream is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children under the age of 12 have not been established.
CLENIA ADVERSE REACTIONS
Although rare, adverse reactions such as dryness, erythema, itching and edema have been reported.
CLENIA DOSAGE AND ADMINISTRATION
CLENIA Foaming Wash
Wash affected areas once or twice daily or as directed by a physician. Wet skin, apply liberally and massage gently into skin for 10-20 seconds working into a full later. Rinse thoroughly and pat dry. Avoid contact with eyes and mucous membranes. If drying occurs, reduce cleansing time and rinse product off sooner or use less often.
CLENIA Emollient Cream
Cleanse skin thoroughly before application. Apply a thin layer to affected areas 1-3 times daily or as directed by a physician. To minimize potential dryness, start with one application daily, then gradually increase to 2-3 times daily as needed or as directed by a physician.
HOW SUPPLIED
CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Foaming Wash is available in 6 oz (170 g) bottles (NDC 0245-0168-06) and 12 oz (340 g) bottles (NDC 0245-0168-12).
CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Emollient Cream is available in 1 oz (28 g) tubes (NDC 0245-0169-01).
Store at 15-25°C (59-77°F). Avoid excessive heat.
Manufactured for
UPSHER-SMITH LABORATORIES, INC.
Minneapolis, MN 55447
by: Contract Pharmaceuticals Limited Canada
Mississauga, Ontario L5N 6L6
CANADA
US Patent 6,977,081 for CLENIA® (sodium sulfacetamide 10% and sulfur 5%) Emollient Cream
100556-01
Revised 0808
2004618
PRINCIPAL DISPLAY PANEL - 28 g tube label
NDC 0245-0169-01
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
1 oz (28 g)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL - 28 g tube carton
NDC 0245-0169-01
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
1 oz (28 g)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL - 3 g sample tube label
NDC 0245-0169-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
3 g (0.1 oz)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL - 3 g sample tube carton
NDC 0245-0169-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Emollient Cream
20 tubes of 3 g (0.1 oz) each
PROFESSIONAL SAMPLES: Not for Sale
UPSHER-SMITH
PRINCIPAL DISPLAY PANEL - 170 g bottle label
NDC 0245-0168-06
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
6 oz (170 g)
UPSHER-SMITH
2003765
R0505

PRINCIPAL DISPLAY PANEL - 170 g bottle carton
NDC 0245-0168-06
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
6 oz (170 g)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL - 340 g bottle label
NDC 0245-0168-12
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
12 oz (340 g)
UPSHER-SMITH
2003771
R0505


PRINCIPAL DISPLAY PANEL - 340 g bottle carton
NDC 0245-0168-12
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
12 oz (340 g)
UPSHER-SMITH

PRINCIPAL DISPLAY PANEL - 5 g sample bottle label
NDC 0245-0168-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
5 g (0.2 oz)
UPSHER-SMITH
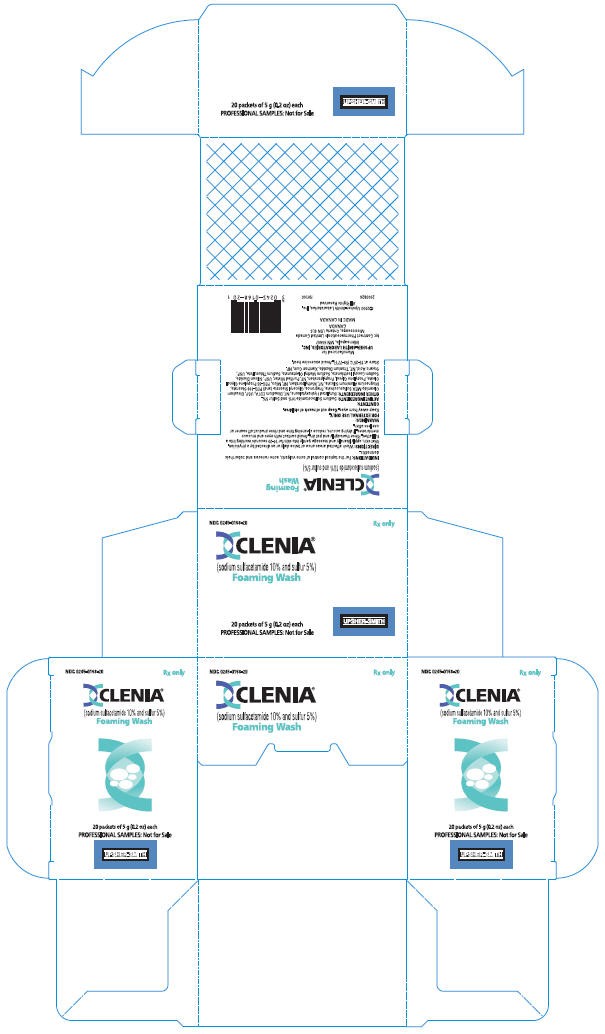
PRINCIPAL DISPLAY PANEL - 5 g sample bottle carton
NDC 0245-0168-20
Rx only
CLENIA®
(sodium sulfacetamide 10% and sulfur 5%)
Foaming Wash
20 packets of 5 g (0.2 oz) each
PROFESSIONAL SAMPLES: Not for Sale
UPSHER-SMITH
Cleniasulfacetamide sodium and sulfur CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cleniasulfacetamide sodium and sulfur CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||