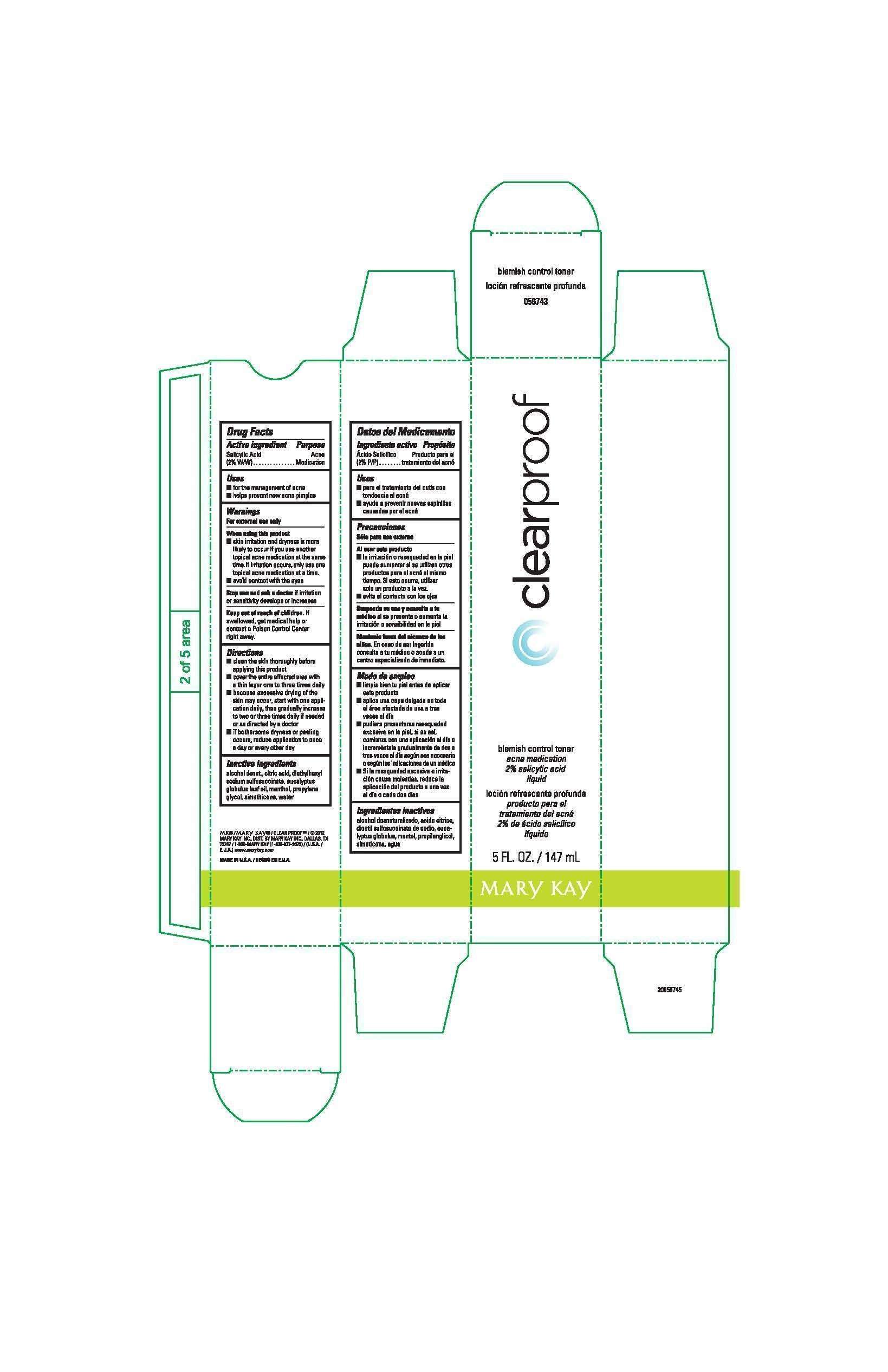
Clear Proof Blemish Control Toner
Clear Proof Blemish Control TonerDrug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Salicylic Acid (2% W/W)
Acne Medication
- for the management of acne
- helps prevent new acne pimples
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid contact with the eyes
Stop use and ask a doctor
if irritation or sensitivity develops or increases
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
alcohol denat., citric acid, diethylhexyl sodium sulfosuccinate, eucalyptus globulus leaf oil, menthol, propylene glycol, simethicone, water
Principal Display Panel - 147 mL carton
clearproof
blemish control toner
acne medication
2% salicylic acid
liquid
5 FL. OZ. / 147 mL
Mary Kay
Clear Proof Blemish Control TonerSalicylic Acid LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!