clear days ahead
Philosophy Inc.
Philosophy Inc.
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
Salicylic Acid 1.0%
Purpose
Acne Treatment
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Uses
- For the treatment of acne.
For external use only
Ask a doctor or pharmacist before using if you are using other topical acne medications at the same time or immediately following use of this product. This may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
When using this product
- avoid contact with eyes. If contact occurs, flush thoroughly with water.
water, sodium, c14-16 olefin sulfonate, acrylates crosspolymer-4, cocomidopropyl-betaine, polysorbate 20, niacinamide, glycerin, panthenol, citrus aurantium dulcis (orange) peel extract, hydrolyzed jojoba esters, jojoba esters, disodium capryloyl glutamate, sodium chloride, sodium sulfate, potassium chloride, butylene glycol, citric acid, sodium hydroxide, hydrogen peroxide, propylene glycol, methylchloroisthiazolinone, methylisothiazolinone, o-cymen-5-ol.

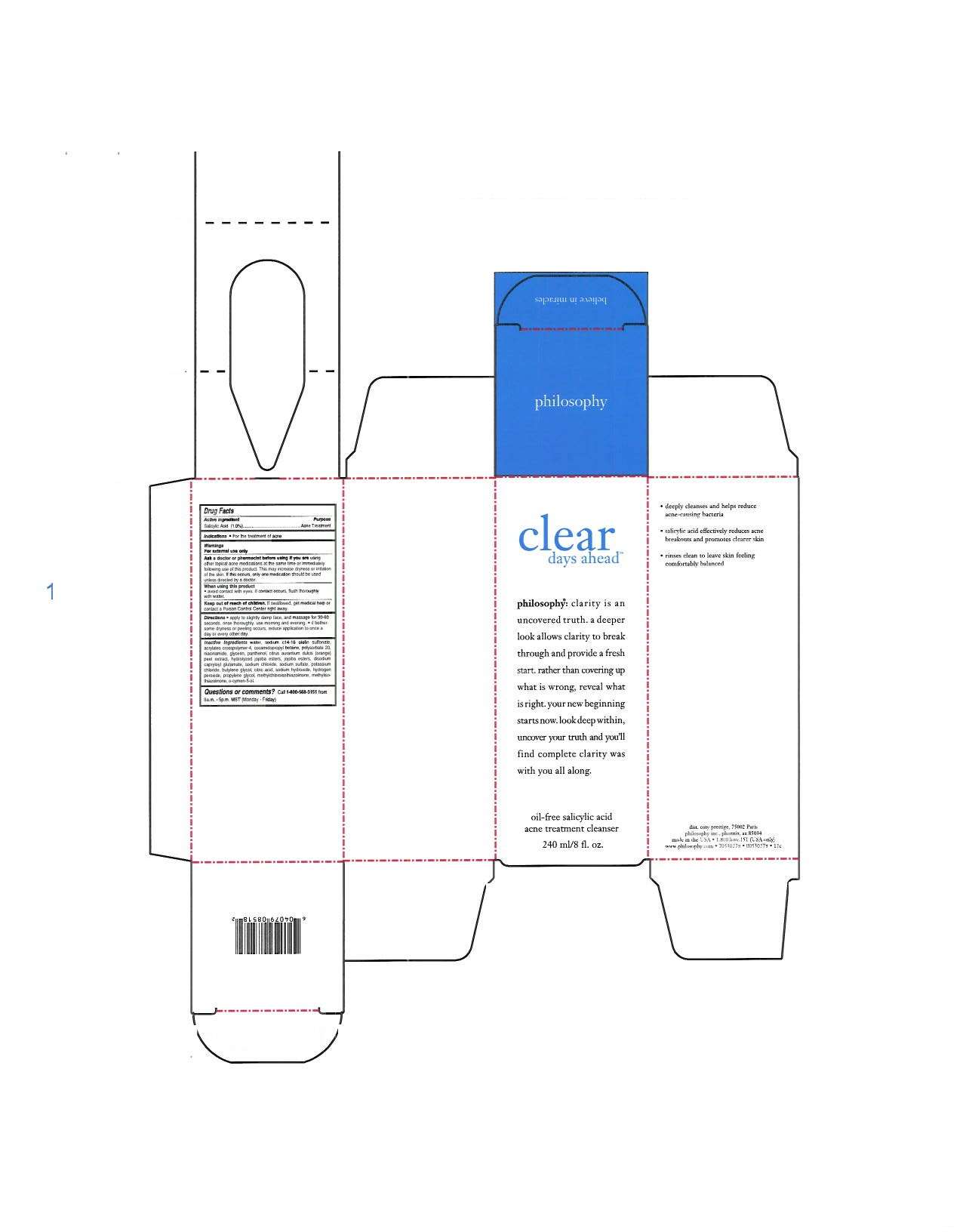
clear days aheadSalicylic Acid LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||