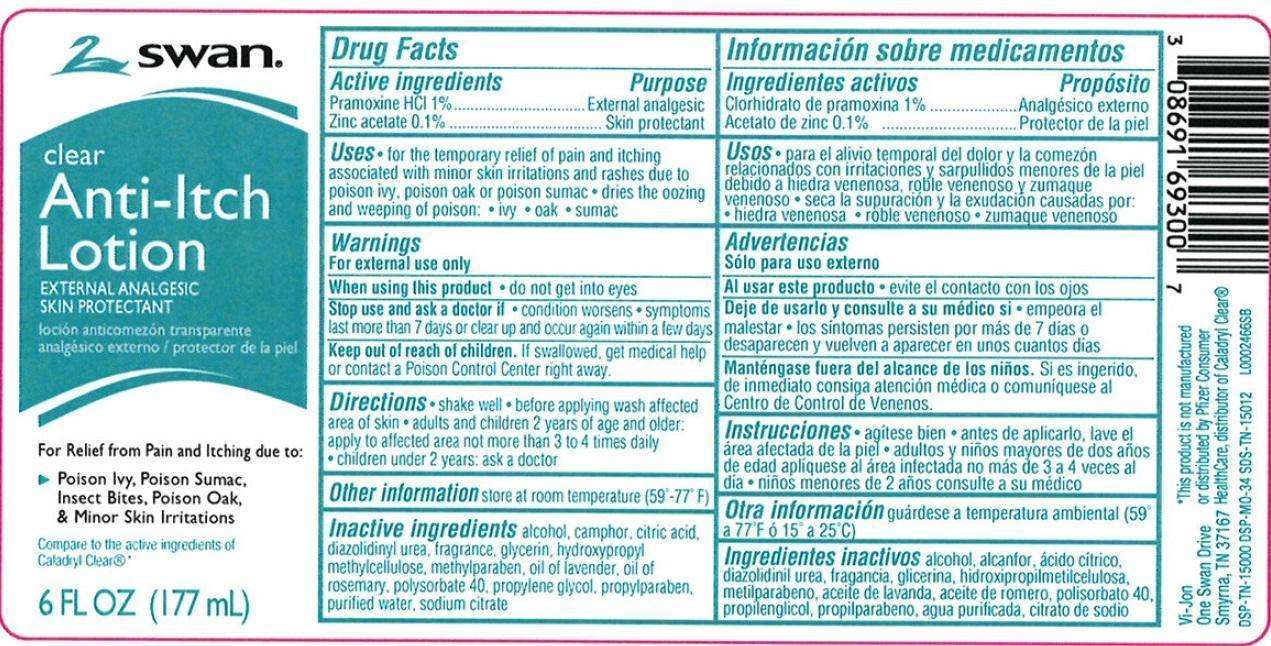
Clear Anti Itch
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Use
- Warnings
- when using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- other information
- Inactive ingredients
- Disclaimer
- Adverse reactions
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredients
Pramoxind HCl 1%
Zinc acetate 0.1%
Purpose
External analgesic
Skin protectant
Use
- for the temporary relief of pain and itching associated with minor skin irritations and rashes due to poison ivy, poison oak or poison sumac
- dries the oozing and weeping of poison:
- ivy
- oak
- sumac
Warnings
For external use only
when using this product
do not get into eyes
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake well
- before applying was affected are of skin
adults and children 2 years of age and older - apply to affected area not more than 3 to 4 times daily
children under 2 years of age - ask a doctor
other information
store at room temperature (59°-77°F)
Inactive ingredients
alcohol, camphor, citric acid, diazolidinyl urea, fragrance, glycerin, hypromellose, methylparaben, oil of lavender, oil of rosemary, polysorbate 40, propylene glycol, propylparaben, purified water, sodium citrate
Disclaimer
This product is not manufactured or distributed by Pfizer Consumer HealthCare, distributor of Caladryl Clear
DSP-TN-15000
DSP-MO-34
SDS-TN-15012
Adverse reactions
Vi-Jon
One Swan Drive
Smyrna, TN 37167
Principal Display Panel
SWAN
Clear
Anti-itch
Lotion
EXTERNAL ANALGESIC
SKIN PROTECTANT
For Relief from Pain and Itchind due to:
Poison Ivy, Poison Sumac, Insect Bites, Poison Oak, + Minor Skin Irritatins
Compare to the active ingredients of Caladryl Clear
6 FL OZ (177 mL)
Clear Anti ItchPramoxine HCl, Zinc acetate LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||