CLEAN AND POLISH LUXURY
FULL PRESCRIBING INFORMATION
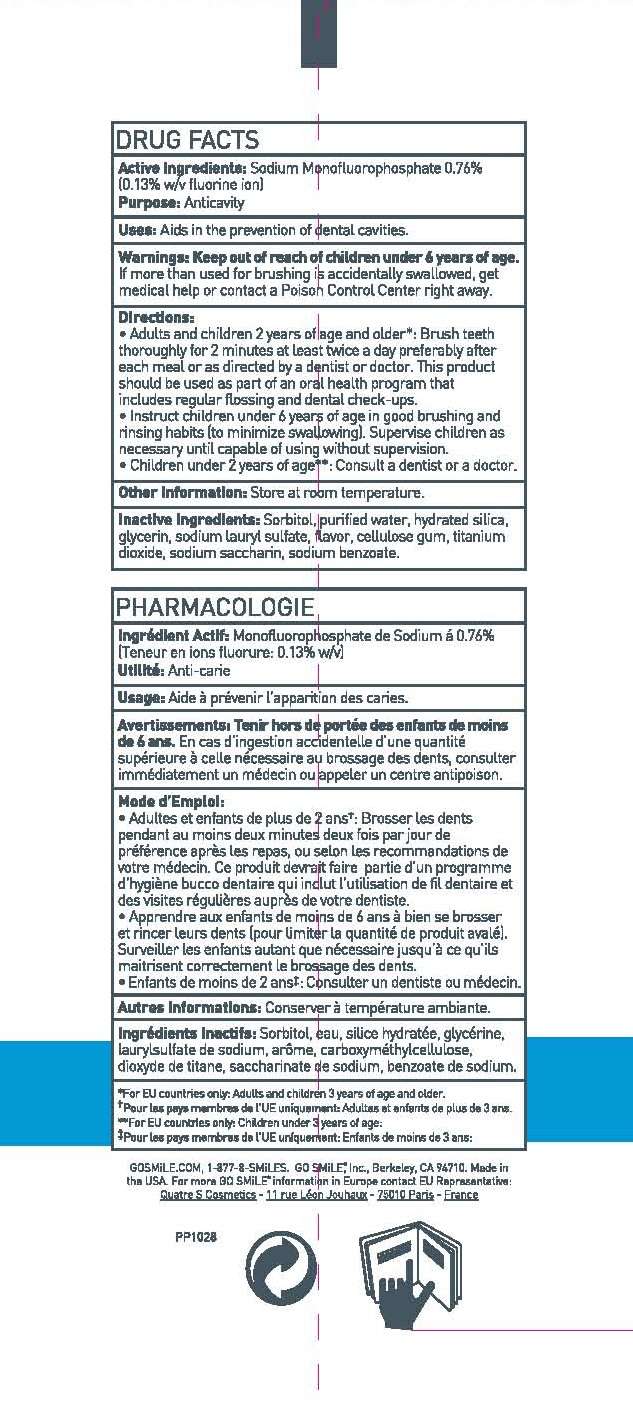
Uses
USES: AIDS IN THE PREVENTION OF DENTAL CAVITIES.
- ADULTS AND CHILDREN 2 YEARS OF AGE AND OLDER*: BRUSH TEETH THOROUGHLY FOR TWO MINUTES AT LEAST TWICE A DAY PREFERABLY AFTER EACH MEAL OR AS DIRECTED BY A DOCTOR OR DENTIST. THIS PRODUCT SHOULD BE USED AS PART OF AN ORAL HEALTH PROGRAM THAT INCLUDES REGULAR FLOSSING AND DENTAL CHECK-UPS.
- INSTRUCT CHILDREN UNDER 6 YEARS OF AGE IN GOOD BRUSHING AND RINSING HABITS (TO MINIMIZE SWALLOWING). SUPERVISE CHILDREN AS NECESSARY UNTIL CAPABLE OF USING WITHOUT SUPERVISION.
- CHILDREN UNDER TWO YEARS OF AGE**: CONSULT A DENTIST OR A DOCTOR.
INACTIVE INGREDIENTS: SORBITOL, PURIFIED WATER, HYDRATED SILICA, GLYCERIN, SODIUM LAURYL SULFATE, FLAVOR, CELLULOSE GUM, TITANIUM DIOXIDE, SODIUM SACCHARIN, SODIUM BENZOATE.
OTHER INFORMATION: STORE AT ROOM TEMPERATURE.
Active ingredient
ACTIVE INGREDIENTS: SODIUM MONOFLUOROPHOSPHATE 0.76% (0.13% w/v FLUORINE ION)
Purpose
PURPOSE: ANTICAVITY
WARNINGS:
IF MORE THAN USED FOR BRUSHING IS ACCIDENTALLY SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.

CLEAN AND POLISH LUXURYSODIUM MONOFLUOROPHOSPHATE PASTE, DENTIFRICE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!