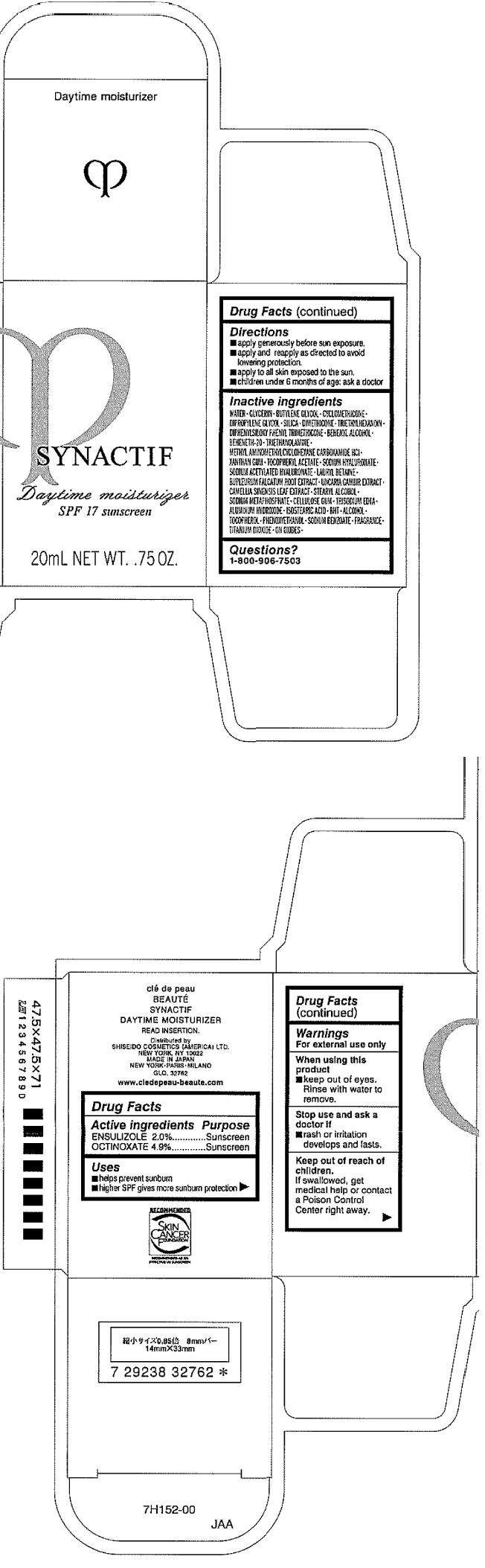
CLE DE PEAU BEAUTE SYNACTIF DAYTIME MOISTURIZER
cle de peau BEAUTE SYNACTIF DAYTIME MOISTURIZER
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLE DE PEAU BEAUTE SYNACTIF DAYTIME MOISTURIZER Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 20mL Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| ENSULIZOLE 2.0% | Sunscreen |
| OCTINOXATE 4.9% | Sunscreen |
CLE DE PEAU BEAUTE SYNACTIF DAYTIME MOISTURIZER Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply generously before sun exposure and as needed.
- apply and reapply as directed to avoid lowering protection.
- apply to all skin exposed to the sun.
- children under 6 months of age: ask a doctor
Inactive ingredients
WATER, GLYCERIN, BUTYLENE GLYCOL, CYCLOMETHICONE, DIPROPYLENE GLYCOL, SILICA, DIMETHICONE, TRIETHYLHEXANOIN, DIPHENYLSILOXY PHENYL TRIMETHICONE, BEHENYL ALCOHOL, BEHENETH-20, TRIETHANOLAMINE, METHYL AMINOMETHYLCYCLOHEXANE CARBOXAMIDE HCl, XANTHAN GUM, TOCOPHERYL ACETATE, SODIUM HYALURONATE, SODIUM ACETYLATED HYALURONATE, LAURYL BETAINE, BUPLEURUM FALCATUM ROOT EXTRACT, UNCARIA GAMBIR EXTRACT, CAMELLIA SINENSIS LEAF EXTRACT, STEARYL ALCOHOL, SODIUM METAPHOSPHATE, CELLULOSE GUM, TRISODIUM EDTA, ALUMINUM HYDROXIDE, ISOSTEARIC ACID, BHT, ALCOHOL, TOCOPHEROL, PHENOXYETHANOL, SODIUM BENZOATE, FRAGRANCE, TITANIUM DIOXIDE, IRON OXIDES
Questions?
1-800-906-7503
Distributed by
SHISEIDO COSMETICS (AMERICA) LTD.
NEW YORK, NY 10022
PRINCIPAL DISPLAY PANEL - 20mL Bottle Carton
SYNACTIF
Daytime moisturizer
SPF 17 sunscreen
20mL NET WT. .75 OZ.
CLE DE PEAU BEAUTE SYNACTIF DAYTIME MOISTURIZEREnsulizole and Octinoxate CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||