Clarithromycin
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLARITHROMYCIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- MICROBIOLOGY
- INDICATIONS & USAGE
- CLARITHROMYCIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CLARITHROMYCIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
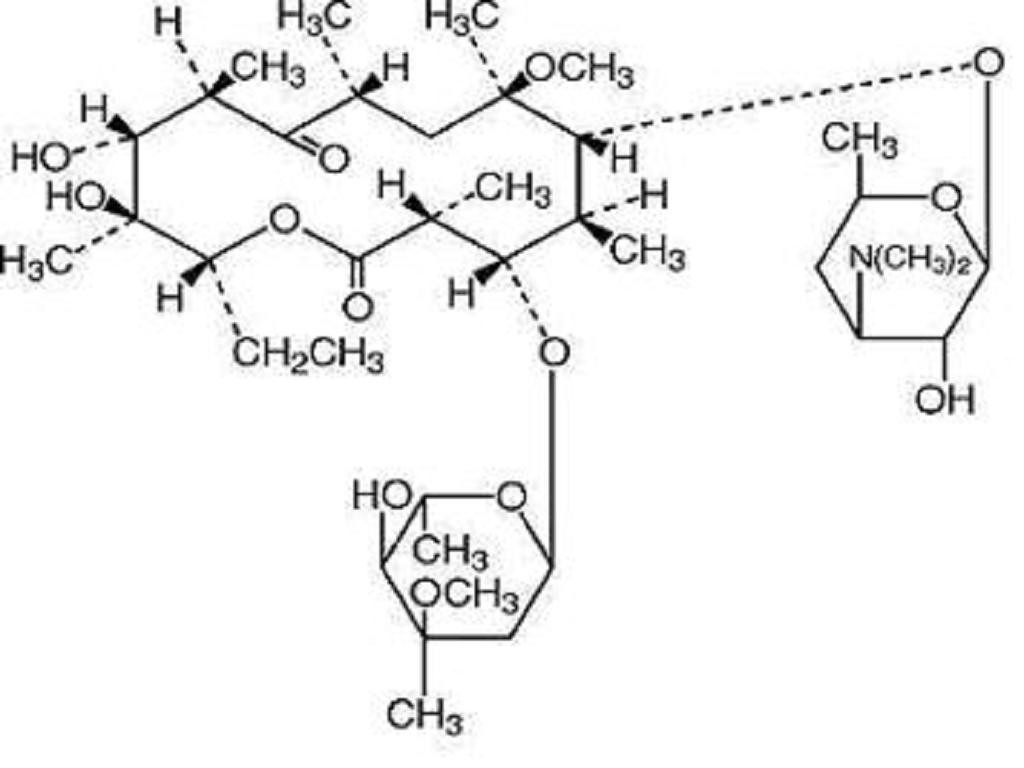
CLARITHROMYCIN DESCRIPTION
DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
PharmacokineticsMICROBIOLOGY
Pretreatment Resistance
*
*
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes
Susceptibility Testing Excluding Mycobacteria and Helicobacter
Dilution Techniques
*
*
*
*
**
Diffusion Techniques
*
*
*
*
**
In Vitro Activity of Clarithromycin Against Mycobacteria
Susceptibility Testing for Mycobacterium avium Complex (MAC)
Susceptibility Test for Helicobacter pylori
***
**
INDICATIONS & USAGE
Adults
Children
Prophylaxis
CLARITHROMYCIN CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
General
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy category C
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CLARITHROMYCIN ADVERSE REACTIONS
Postmarketing Experience
Changes in Laboratory Values
OVERDOSAGE
DOSAGE & ADMINISTRATION
ClarithromycinTabletsInfection
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy: Clarithromycin/Lansoprazole/Amoxicillin
Triple Therapy: Clarithromycin/Omeprazole/Amoxicillin
Dual Therapy: Clarithromycin/Omeprazole
Dual Therapy: Clarithromycin/Ranitidine Bismuth Citrate
Children
Based on Body WeightDosing Calculated on 7.5 mg/kg q12hWeightDoseKglbs(q12h)125 mg/5 mL250 mg/5 mL
Mycobacterial Infections
Prophylaxis
Treatment
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

ClarithromycinClarithromycin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!