CITOMIX
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS/PURPOSE
ANANASSA 3X IMMUNE SUPPORT
GCSF 4C, 9C, 15C, 30C IMMUNE SUPPORT
HYDROCOTYLE ASIATICA 3X INFLAMMATION
INTERFERON GAMMA 4C IMMUNE SUPPORT
INTERLEUKIN 1 BETA 5C IMMUNE SUPPORT
INTERLEUKIN 2 5C, 7C FEVER REDUCER
INTERLEUKIN 4 4C FEVER REDUCER
INTERLEUKIN 6 7C, 9C, 15C IMMUNE SUPPORT
LYMPHATIC VESSEL 4C DETOXIFICATION
MEDULLA OSSIS 4C DETOXIFICATION
MOUNTAIN CRANBERRY 3X DECONGESTION
THYMUS GLAND 4C IMMUNE SUPPORT
USES
For Immune Support during times of seasonal colds and flu
WARNINGS
Stop use and ask doctor if cold and flu-like symptoms such as fever, sore throat, headache, minor aches and pain worsen or persist more than 5 days
PREGNANCY
If pregnant or breast-feeding ask a health professional before use.
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 5 pellets 3 times per day
Children between 12 years and 6 years of age 3 pellets 3 times per day
Children under 6 years 1 pellet 3 times per day to be dissolved into a little water
QUESTIONS
Questions?: info@gunainc.com
tel. (484) 223-3500
PRINCIPAL DISPLAY PANEL
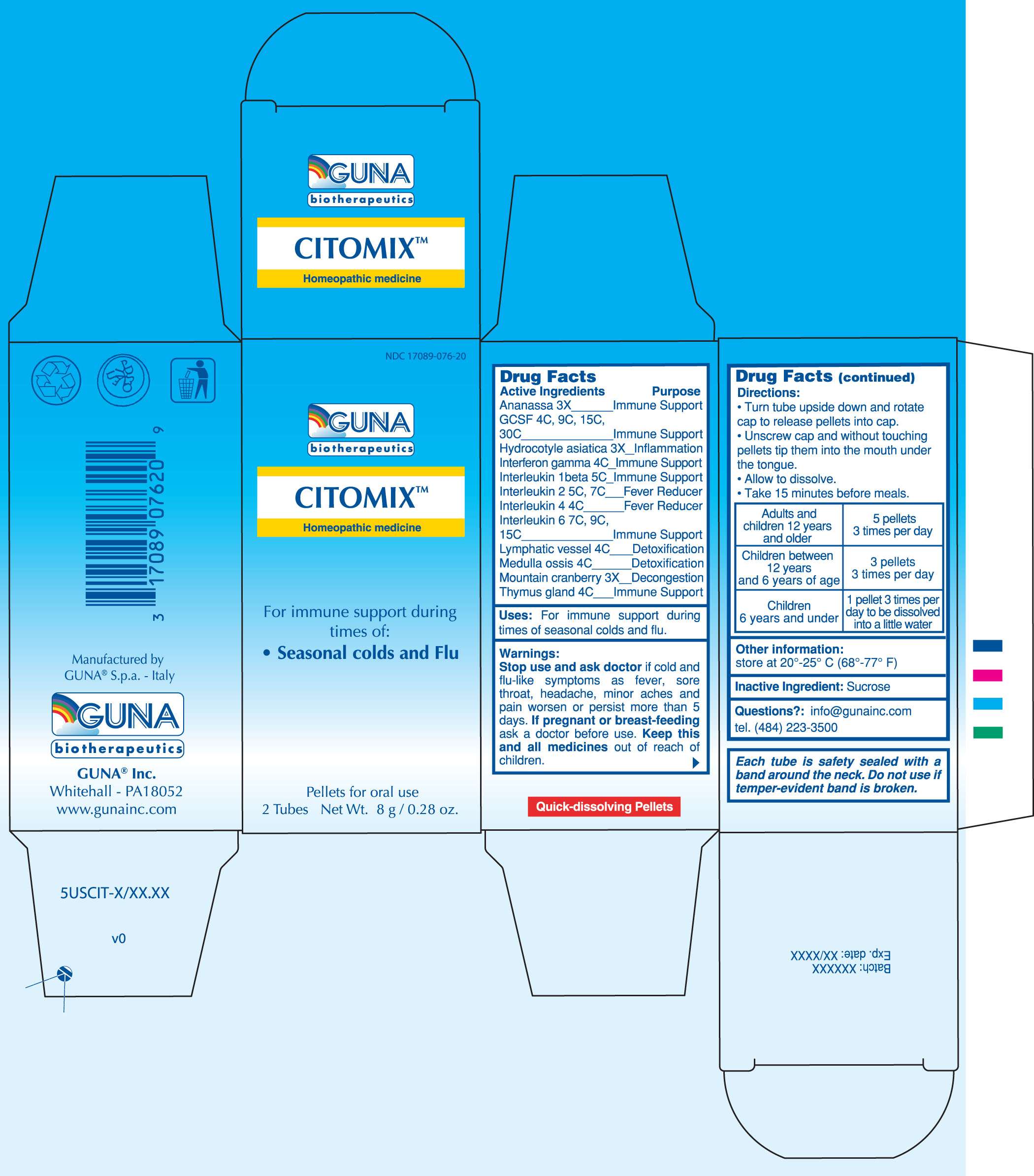
CITOMIXALDESLEUKIN - BINETRAKIN - CANAKINUMAB - CENTELLA ASIATICA - CRANBERRY - HUMAN INTERLEUKIN-6 (NONGLYCOSYLATED) - INTERFERON GAMMA-1B - LENOGRASTIM - PINEAPPLE - SUS SCROFA BONE MARROW - SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE - SUS SCROFA THYMUS - PELLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||