Citalopram Hydrobromide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CITALOPRAM HYDROBROMIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CITALOPRAM HYDROBROMIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CITALOPRAM HYDROBROMIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- SPL MEDGUIDE
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsWARNINGS: Clinical Worsening and Suicide RiskPRECAUTIONS: Information for PatientsPRECAUTIONS: Pediatric Use
CITALOPRAM HYDROBROMIDE DESCRIPTION
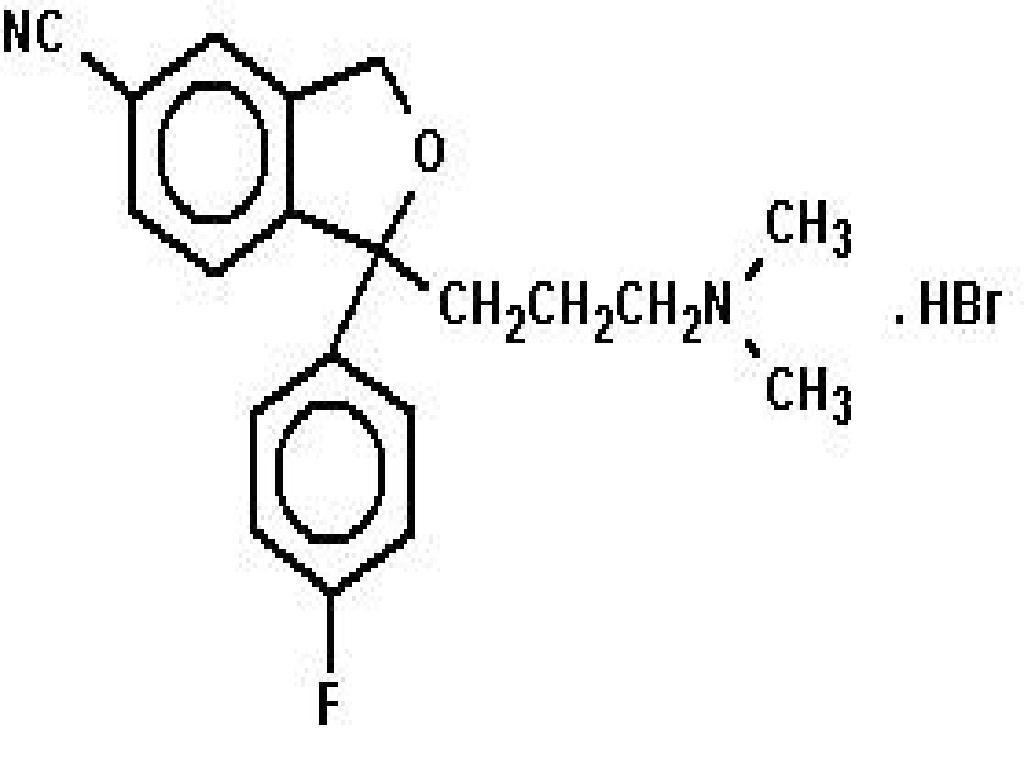
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Absorption and Distribution
Metabolism and Elimination
Population Subgroups
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
Drug-Drug Interactions
Drug InteractionsPRECAUTIONS
Clinical Efficacy Trials
Comparison of Clinical Trial Results
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
CITALOPRAM HYDROBROMIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONS
WARNINGS
Clinical Worsening and Suicide RiskAge RangeDrug-Placebo Difference in Number of Cases of Suicidalityper 1000 Patients Treated
PRECAUTIONSDOSAGE AND ADMINISTRATIONDiscontinuation of Treatment with Citalopram Tablets
Screening Patients for Bipolar Disorder
Potential for Interaction with Monoamine Oxidase Inhibitors
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
PRECAUTIONS
GeneralDiscontinuation of Treatment with Citalopram Tablets
DOSAGE AND ADMINISTRATION
Abnormal Bleeding
Hyponatremia
Geriatric Use
Activation of Mania/Hypomania
Seizures
Interference with Cognitive and Motor Performance
Use in Patients with Concomitant Illness
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
WARNINGS-Serotonin SyndromePRECAUTIONS - Drug Interactions
WARNINGS - Serotonin Syndrome
CONTRAINDICATIONSWARNINGS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Nonteratogenic Effects
WARNINGS
DOSAGE AND ADMINISTRATION
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNINGWARNINGS-Clinical Worsening and Suicide RiskGERIATRIC USE
DOSAGE AND ADMINISTRATIONPRECAUTIONS, Hyponatremia
CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
CITALOPRAM HYDROBROMIDE ADVERSE REACTIONS
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials
Adverse Events Associated with Discontinuation of Treatment
Body System/Adverse EventPercentage of Patients DiscontinuingDue to Adverse EventCitalopramPlacebo(N = 1063)(N = 446)GeneralGastrointestinal DisordersCentral and Peripheral Nervous System DisordersPsychiatric Disorders
Adverse Events Occurring at an Incidence of 2% or More Among Citalopram-Treated Patients
Body System/Adverse Event(Percentage of Patients Reporting Event)Citalopram TabletsPlacebo(N = 1063)(N = 446)Autonomic Nervous System DisordersCentral & Peripheral Nervous System DisordersGastrointestinal DisordersGeneralMusculoskeletal System DisordersPsychiatric DisorderRespiratory System DisordersUrogenital
Dose Dependency of Adverse Events
Male and Female Sexual Dysfunction with SSRIs
TreatmentCitalopram Tablets (Placebo(425 males)(194 males)
Vital Sign Changes
Weight Changes
Laboratory Changes
ECG Changes
Other Events Observed During the Premarketing Evaluation of Citalopram Tablets
ADVERSE REACTIONS
Other Events Observed During the Postmarketing Evaluation of Citalopram Tablets
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceManagement of Overdose
DOSAGE & ADMINISTRATION
Initial TreatmentSpecial Populations
Treatment of Pregnant Women During the Third Trimester
PRECAUTIONS
Maintenance Treatment
Clinical TrialsCLINICAL PHARMACOLOGY
Discontinuation of Treatment with Citalopram Tablets
PRECAUTIONS
Switching Patients To or From a Monoamine Oxidase Inhibitor
CONTRAINDICATIONSWARNINGS
HOW SUPPLIED
STORAGE AND HANDLING
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
ANIMAL TOXICOLOGYRetinal Changes in Rats
Cardiovascular Changes in Dogs
SPL MEDGUIDE
Medication Guide-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
●
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
●
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
●
-
● Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
-
●
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:COPOVIDONE
STARCH, CORN
CROSCARMELLOSE SODIUM
LACTOSE MONOHYDRATE
MAGNESIUM STEARATE
HYPROMELLOSE 2910 (6 MPA.S)
CELLULOSE, MICROCRYSTALLINE
POLYETHYLENE GLYCOL 400
TITANIUM DIOXIDEF
ERRIC OXIDE RED
FERRIC OXIDE YELLOW
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Citalopram HydrobromideCitalopram Hydrobromide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!