Chlorpromazine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CHLORPROMAZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CHLORPROMAZINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CHLORPROMAZINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
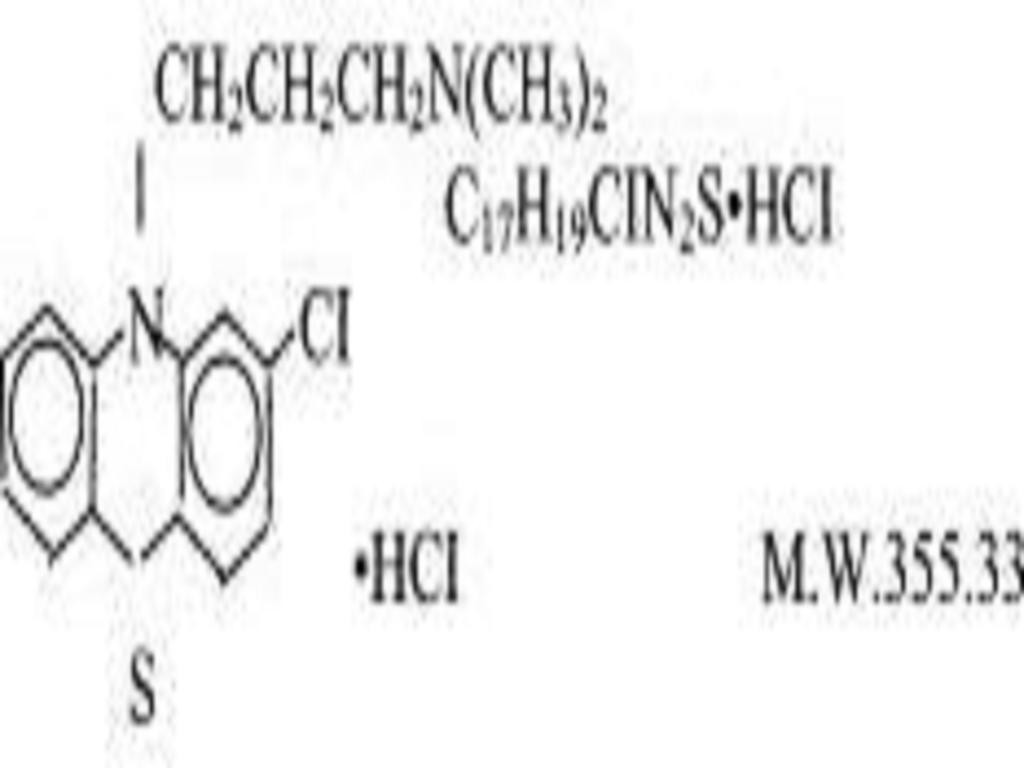
CHLORPROMAZINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
CHLORPROMAZINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related PsychosisBOXED WARNING
The extrapyramidal symptoms which can occur secondary to chlorpromazine may be confused with the central nervous system signs of an undiagnosed primary disease responsible for the vomiting, e.g., Reye's syndrome or other encephalopathy. The use of chlorpromazine and other potential hepatotoxins should be avoided in children and adolescents whose signs and symptoms suggest Reye's syndrome.
Tardive Dyskinesia:
PRECAUTIONSADVERSE REACTIONS
Neuroleptic Malignant Syndrome (NMS):
Usage in Pregnancy:
Non-teratogenic Effects:
Nursing Mothers:
PRECAUTIONS
Leukopenia, Neutropenia and AgranulocytosisGeneral
Note:notnot
Long-Term Therapy:
Antiemetic Effect:WARNINGS
Abrupt Withdrawal:
CHLORPROMAZINE HYDROCHLORIDE ADVERSE REACTIONS
Note:Drowsiness:
Jaundice:
Hematological Disorders:
Agranulocytosis
Cardiovascular:
Hypotensive Effects
EKG Changes
Note:
CNS Reactions:
Dystonia
Class effect
Motor Restlessness:
Pseudoparkinsonism:
Tardive Dyskinesia:
Adverse Behavioral Effects
Other CNS EffectsWARNINGS
Allergic Reactions
Endocrine Disorders:
Autonomic Reactions:
Special Considerations In Long-Term Therapy
Skin Pigmentation
Ocular Changes
Etiology
Other Adverse Reactions:
Note:
OVERDOSAGE
ADVERSE REACTIONSSymptoms
Treatment
DOSAGE & ADMINISTRATION
ADULTSPEDIATRIC PATIENTS (6 months to 12 years of age)
Severe Behavioral Problems
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Chlorpromazine HydrochlorideChlorpromazine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!