Childrens Acetaminophen
CHILDREN's ACETAMINOPHEN ORAL SUSPENSION
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient(in each 5 mL)
- Purpose
- Childrens Acetaminophen Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- How Supplied
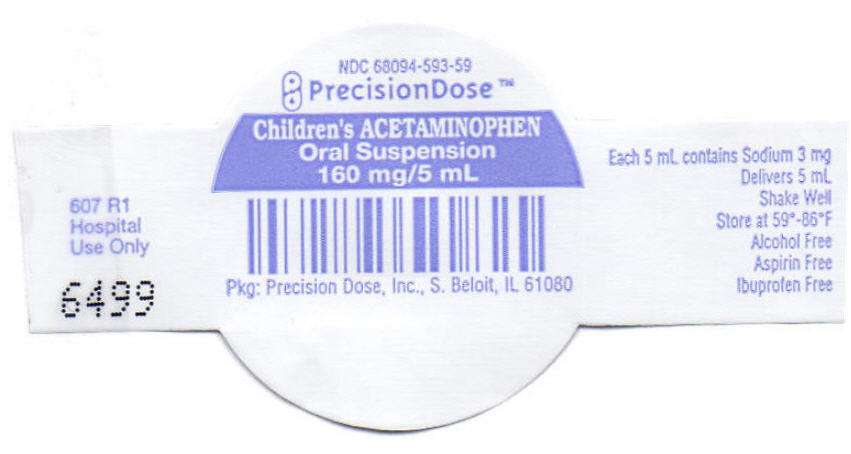
- PRINCIPAL DISPLAY PANEL - 160 mg/5 mL Cup Lid
- PRINCIPAL DISPLAY PANEL - 325 mg/10.15 mL Cup Lid
- PRINCIPAL DISPLAY PANEL - 650 mg/20.3 mL Cup Lid
FULL PRESCRIBING INFORMATION
160 mg/5 mL
325 mg/10.15 mL
650 mg/20.3 mL
Grape Flavor
For Hospital Use Only
Drug Facts
Active Ingredient(in each 5 mL)
Acetaminophen 160 mg
Purpose
Pain reliever/Fever reducer
Childrens Acetaminophen Uses
Temporarily relieves minor aches and pains due to:
- the common cold
- flu
- headaches
- sore throat
- immunizations
- toothaches
- reduces fever
Warnings
Overdose warning
Taking more than the recommended dose can cause serious health problems, including liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical even if you do not notice any signs or symptoms.
Do not use with any other product containing acetaminophen.
Stop use and ask a doctor if
- new symptoms occur
- redness or swelling is present
- pain gets worse or lasts for more than 5 days
- fever gets worse or lasts for more than 3 days
- sore throat is severe, lasts for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting
Keep out of reach of children.
Directions
- Use as directed per healthcare professional.
- do not use more than directed (see overdose warning)
- shake cups well before using
- repeat dose every 4 hours, if needed
- do not use more than 5 times in 24 hours
Other Information
- each teaspoon contains: sodium 3 mg
- store at room temperature 15° - 30°C (59° - 86°F)
- This product is not the same concentration as Infant's Drops. Use as directed per healthcare professional.
- see individual label or shipper label for lot number and expiration date
Inactive Ingredients
butylparaben, carboxymethylcellulose sodium, carrageenan, citric acid, D&C red no. 33, FD&C blue no. 1, flavors, glycerin, high fructose corn syrup, hydroxyethyl cellulose, microcrystalline cellulose, propylene glycol, purified water, sodium benzoate, sorbitol
Alcohol Free, Aspirin Free, Ibuprofen Free
How Supplied
NDC 68094-593-58
5 mL per unit dose syringe
Fifty (50) unit dose syringes per shipper
NDC 68094-593-61
5 mL per unit dose cup
One hundred (100) cups per shipper
NDC 68094-593-62
5 mL per unit dose cup
Thirty (30) cups per shipper
NDC 68094-614-62
10.15 mL per unit dose cup
Thirty (30) cups per shipper
NDC 68094-650-62
20.3 mL per unit dose cup
Thirty (30) cups per shipper
Mfg. By: Perrigo
515 Eastern Avenue
Allegan, MI 49010
Pkg. By: Precision Dose, Inc.
722 Progressive Lane
S. Beloit, IL 61080
LI806
Rev. 02/10
PRINCIPAL DISPLAY PANEL - 160 mg/5 mL Cup Lid
NDC 68094-593-59
PrecisionDose™
Children's ACETAMINOPHEN
Oral Suspension
160 mg/5 mL
Pkg: Precision Dose, Inc., S. Beloit, IL 61080
PRINCIPAL DISPLAY PANEL - 325 mg/10.15 mL Cup Lid
NDC 68094-614-59
PrecisionDose™
Children's ACETAMINOPHEN
Oral Suspension
325 mg/10.15 mL
Delivers 10.15 mL Shake Well
Each 5 mL contains Sodium 3 mg
Alcohol Free Aspirin Free Ibuprofen Free
Store at Room Temp (59°-86°F)
Pkg. By: Precision Dose, Inc.
S. Beloit, IL 61080

PRINCIPAL DISPLAY PANEL - 650 mg/20.3 mL Cup Lid
NDC 68094-650-59
PrecisionDose™
Children's ACETAMINOPHEN
Oral Suspension
650 mg/20.3 mL
Delivers 20.3 mL Shake Well
Each 5 mL contains Sodium 3 mg
Alcohol Free Aspirin Free Ibuprofen Free
Store at Room Temp (59°-86°F)
Pkg. By: Precision Dose, Inc.
S. Beloit, IL 61080

Childrens AcetaminophenAcetaminophen SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Childrens AcetaminophenAcetaminophen SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Childrens AcetaminophenAcetaminophen SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||