Cetaphil DermaControl SPF 30
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients......Purpose
- Cetaphil DermaControl SPF 30 Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Principle Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients......Purpose
Cetaphil DermaControl SPF 30 Uses
Helps Prevent Sunburn
If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only, not to be swallowed
- Do not use on damaged or broken skin.
- Stop use and ask a doctor if rash occurs.
- When using this product keep out of eyes. Rinse with water to remove.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
Other Information
Protect this product from excessive heat and direct sun.
Inactive Ingredients
Water, Isopropyl Lauroyl Sarcosinate, Glycerin, Dimethicone, Diisopropyl Sebacate, Silica, Polymethyl Methacrylate, Aluminum Starch Octenylsuccinate, Sucrose Tristeatrate, Dimethiconol, Pentylene Glycol, Polysorbate 61, Sodium Stearoyl Glutamate, Phenoxyethanol, Caprylyl Glycol, Tocopheryl Acetate, Glycyrrhetinic Acid, Panthenol, Triethanolamine, Allantoin, Carbomer, Potassium Sorbate, Zinc Gluconate, Xanthan Gum, Disodium EDTA, Hydroxypalmitoyl Sphinganine
Principle Display Panel
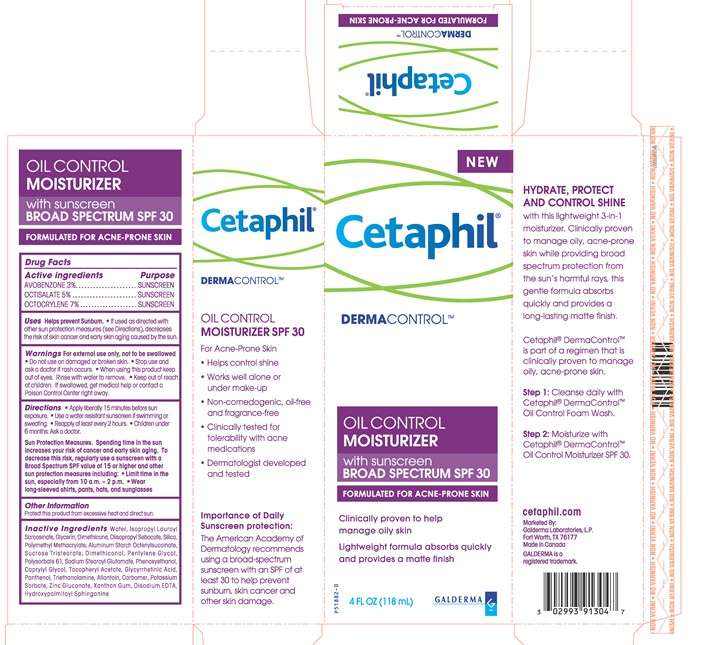
NEW
Cetaphil®
DERMACONTROLTM
OIL CONTROL
MOISTURIZER
with sunscreen
BROAD SPECTRUM SPF 30
FORMULATED FOR ACNE-PRONE SKIN
Clinically proven to help manage oily skin
Lightweight formula absorbs quickly and provides a matte finish
P51882-0
4 FL OZ (118 mL)
GALDERMA
OIL CONTROL
MOISTURIZER SPF 30
For Acne-Prone Skin
- Helps control shine
- Works well alone or under make-up
- Non-comedogenic, oil-free, and fragrance-free
- Clinically tested for tolerability with acne medications
- Dermatologist developed and tested
Importance of Daily Sunscreen protection:
The American Academy of Dermatology recommends using a broad-spectrum sunscreen with an SPF of at least 30 to help prevent sunburn, skin cancer and other skin damage.
HYDRATE, PROTECT AND CONTROL SHINE
with this lightweight 3-in-1 mosturizer. Clinically proven to manage oily, acne-prone skin while providing broad spectrum protection from the sun's harmful rays, this gentle formula absorbs quickly and provides a long-lasting matte finish.
Cetaphil® DermaControlTM is part of a regimen that is clinically proven to manage oily, acne-prone skin.
Step 1: Cleanse daily with Cetaphil® DermaControlTM Oil Control Foam Wash.
Step 2: Moisturize with Cetaphil® DermaControlTM Oil Control Moisterizer SPF 30.
cetaphil.com

NEW
®
TM
MOISTURIZER
BROAD SPECTRUM SPF 30
FORMULATED FOR ACNE-PRONE SKIN
OPEN HERE
MOSTURIZER
SPF30
Works well alone
or under make-up
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours to maintain SPF level.
ACTIVE INGREDIENTS
cetaphil.com
Cetaphil DermaControl SPF 30octocrylene, octisalate, avobenzone LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||