Cepacol
Cēpacol Extra Strength* Sore Throat mixed berry
FULL PRESCRIBING INFORMATION: CONTENTS*
- Cepacol Uses
- Warnings
- Directions
- Cepacol Other information
- Inactive ingredients
- Questions?
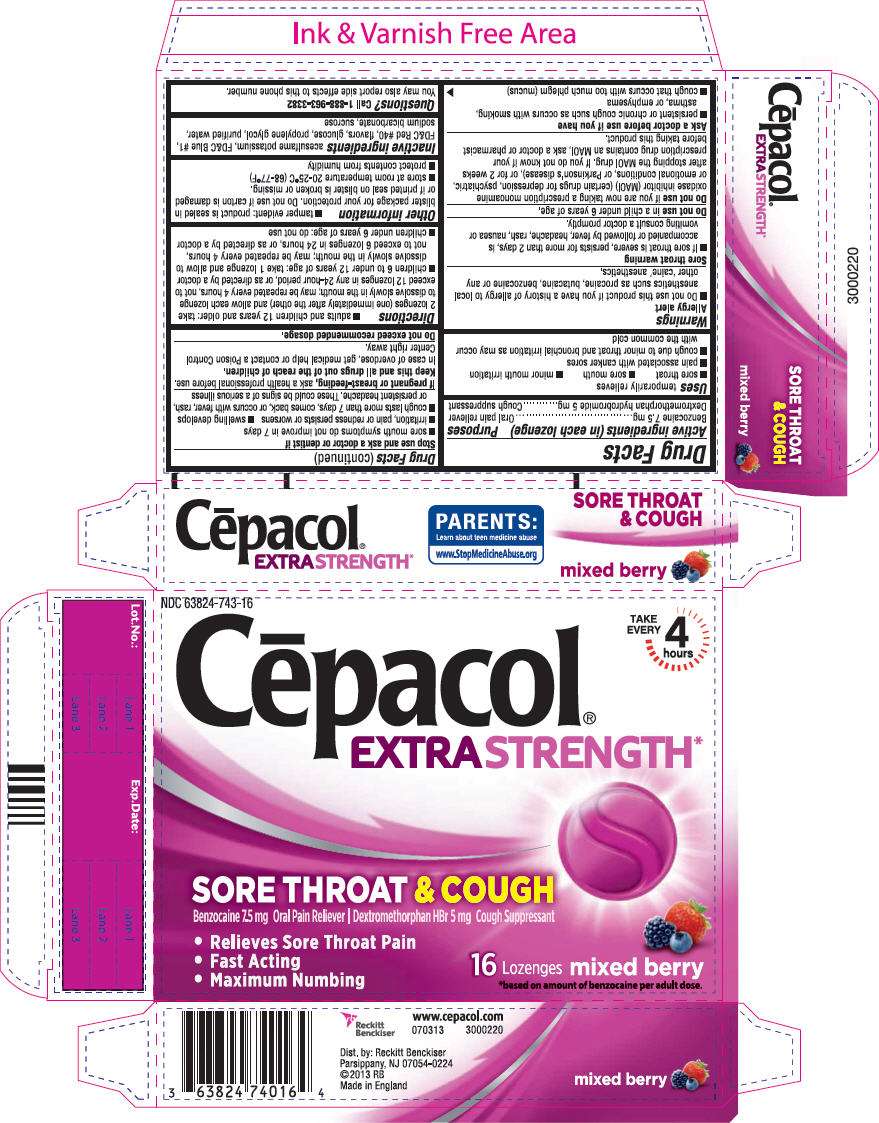
- PRINCIPAL DISPLAY PANEL - 16 Lozenge Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients (in each lozenge) | Purposes |
|---|---|
| Benzocaine 7.5 mg | Oral pain reliever |
| Dextromethorphan hydrobromide 5 mg | Cough suppressant |
Cepacol Uses
temporarily relieves
- sore throat
- sore mouth
- minor mouth irritation
- pain associated with canker sores
- cough due to minor throat and bronchial irritation as may occur with the common cold
Warnings
Allergy alert
- Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning
- If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting consult a doctor promptly.
Do not use in a child under 6 years of age.
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain or redness persists or worsens
- swelling develops
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness
If pregnant or breast-feeding, ask a health professional before use.
Keep this and all drugs out of the reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not exceed recommended dosage.
Directions
- adults and children 12 years and older: take 2 lozenges (one immediately after the other) and allow each lozenge to dissolve slowly in the mouth; may be repeated every 4 hours, not to exceed 12 lozenges in any 24-hour period, or as directed by a doctor
- children 6 to under 12 years of age: take 1 lozenge and allow to dissolve slowly in the mouth; may be repeated every 4 hours, not to exceed 6 lozenges in 24 hours, or as directed by a doctor
- children under 6 years of age: do not use
Cepacol Other information
- tamper evident: product is sealed in blister package for your protection. Do not use if carton is damaged or if printed seal on blister is broken or missing.
- store at room temperature 20-25°C (68-77°F)
- protect contents from humidity
Inactive ingredients
acesulfame potassium, FD&C Blue #1, FD&C Red #40, flavors, glucose, propylene glycol, purified water, sodium bicarbonate, sucrose
Questions?
Call 1-888-963-3382
You may also report side effects to this phone number.
Dist. by: Reckitt Benckiser
Parsippany, NJ 07054-0224
Made in England
PRINCIPAL DISPLAY PANEL - 16 Lozenge Carton
NDC 63824-743-16
Cēpacol®
EXTRA STRENGTH*
TAKE
EVERY
4
hours
SORE THROAT & COUGH
Benzocaine 7.5 mg Oral Pain Reliever | Dextromethorphan HBr 5 mg Cough Suppressant
- Relieves Sore Throat Pain
- Fast Acting
- Maximum Numbing
16
Lozenges
mixed berry
*based on amount of benzocaine per adult dose.
CepacolBENZOCAINE and DEXTROMETHORPHAN HYDROBROMIDE LOZENGE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||