Cefdinir
Northstar Rx LLC
Aurobindo Pharma Limited
Cefdinir Capsules, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFDINIR DESCRIPTION
- CLINICAL PHARMACOLOGY
- CEFDINIR INDICATIONS AND USAGE
- CEFDINIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEFDINIR ADVERSE REACTIONS
- OVERDOSAGE
- CEFDINIR DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- REFERENCES
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (30 Capsule Bottle)
FULL PRESCRIBING INFORMATION
CEFDINIR DESCRIPTION
1413552
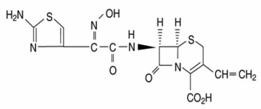
Cefdinir capsules, USP contain 300 mg cefdinir USP and the following inactive ingredients: carboxymethylcellulose calcium, colloidal silicon dioxide and magnesium stearate. The empty hard gelatin capsule shells contain FD&C Blue #1, D&C Red #28, titanium dioxide, gelatin and sodium lauryl sulphate. The capsules are printed with edible ink containing black iron oxide and shellac.
CLINICAL PHARMACOLOGY
Pharmacokinetics and Drug Metabolism
Absorption
Oral Bioavailability
Effect of Food
maxmax
Cefdinir Capsules
| Dose | Cmax
(mcg/mL) |
tmax
(hr) |
AUC (mcg•hr/mL) |
|---|---|---|---|
| 300 mg |
1.6 (0.55) |
2.9 (0.89) |
7.05 (2.17) |
| 600 mg |
2.87 (1.01) |
3 (0.66) |
11.1 (3.87) |
Cefdinir Suspension
| Dose | Cmax
(mcg/mL) |
tmax
(hr) |
AUC (mcg•hr/mL) |
|---|---|---|---|
| 7 mg/kg |
2.3 (0.65) |
2.2 (0.6) |
8.31 (2.5) |
| 14 mg/kg |
3.86 (0.62) |
1.8 (0.4) |
13.4 (2.64) |
Multiple Dosing
Distribution
areaarea
Skin Blister
max(0-∞)
Tonsil Tissue
Sinus Tissue
Lung Tissue
Middle Ear Fluid
CSF
Metabolism and Excretion
½Special Populations: Patients with Renal Insufficiency
DOSAGE AND ADMINISTRATION
Special Populations
Patients with Renal Insufficiency
crcrmax½crmax½ DOSAGE AND ADMINISTRATION
Hemodialysis
½DOSAGE AND ADMINISTRATION
Hepatic Disease
Geriatric Patients
max½Patients with Renal Insufficiency
Gender and Race
Microbiology
in vitro INDICATIONS AND USAGE
Aerobic Gram-Positive Microorganisms
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes
Aerobic Gram-Negative Microorganisms
Haemophilus influenzae
Haemophilus parainfluenzae
Moraxella catarrhalis
in vitro but their clinical significance is unknown
in vitro
Aerobic Gram-Positive Microorganisms
Staphylococcus epidermidis
Streptococcus agalactiae
Enterococcus Staphylococcus
Aerobic Gram-Negative Microorganisms
Citrobacter diversus
Escherichia coli
Klebsiella pneumoniae
Proteus mirabilis
Pseudomonas Enterobacter
Susceptibility Tests
Dilution Techniques
(1)
Haemophilus Streptococcus
| MIC (mcg/mL) | Interpretation |
|---|---|
| ≤1 |
Susceptible (S) |
| 2 |
Intermediate (I) |
| ≥4 |
Resistant (R) |
Haemophilus a
| MIC (mcg/mL) | Interpretationb |
|---|---|
|
a These interpretive standards are applicable only to broth microdilution susceptibility tests with Haemophilus spp. using Haemophilus Test Medium (HTM).(1)
b The current absence of data on resistant strains precludes defining any results other than “Susceptible.” Strains yielding MIC results suggestive of a “nonsusceptible” category should be submitted to a reference laboratory for further testing. |
|
| ≤1 |
Susceptible (S) |
Streptococcus
Streptococcus pneumoniae S. pneumoniae
| Microorganism | MIC Range (mcg/mL) |
|---|---|
|
c This quality control range is applicable only to H. influenzae ATCC 49766 tested by a broth microdilution procedure using HTM. |
|
|
Escherichia coli ATCC 25922 |
0.12-0.5 |
|
Haemophilus influenzae ATCC 49766c
|
0.12-0.5 |
|
Staphylococcus aureus ATCC 29213 |
0.12-0.5 |
Diffusion Techniques
(2)
Haemophilus Streptococcus d
| Zone Diameter (mm) | Interpretation |
|---|---|
|
d Because certain strains of Citrobacter, Providencia, and Enterobacter spp. have been reported to give false susceptible results with the cefdinir disk, strains of these genera should not be tested and reported with this disk. |
|
| ≥20 |
Susceptible (S) |
| 17-19 |
Intermediate (I) |
| ≤16 |
Resistant (R) |
Haemophilus e
| Zone Diameter (mm) | Interpretationf |
|---|---|
|
e These zone diameter standards are applicable only to tests with Haemophilus spp. using HTM.(2)
f The current absence of data on resistant strains precludes defining any results other than “Susceptible.” Strains yielding MIC results suggestive of a “nonsusceptible” category should be submitted to a reference laboratory for further testing. |
|
| ≥20 |
Susceptible (S) |
Streptococcus
Streptococcus pneumoniae S. pneumoniae
| Organism | Zone Diameter (mm) |
|---|---|
|
g This quality control range is applicable only to testing of H. influenzae ATCC 49766 using HTM. |
|
|
Escherichia coli ATCC 25922 |
24-28 |
|
Haemophilus influenzae ATCC 49766g
|
24-31 |
|
Staphylococcus aureus ATCC 25923 |
25-32 |
CEFDINIR INDICATIONS AND USAGE
Adults and Adolescents
Community-Acquired Pneumonia Haemophilus influenzae Haemophilus parainfluenzae Streptococcus pneumoniae Moraxella catarrhalis CLINICAL STUDIES
Acute Exacerbations of Chronic Bronchitis Haemophilus influenzae Haemophilus parainfluenzae Streptococcus pneumoniae Moraxella catarrhalis
Acute Maxillary Sinusitis Haemophilus influenzae Streptococcus pneumoniae Moraxella catarrhalis
NOTE: Pediatric Use DOSAGE AND ADMINISTRATION
Pharyngitis/Tonsillitis Streptococcus pyogenes CLINICAL STUDIES
NOTE: S. pyogenes S. pyogenes
Uncomplicated Skin and Skin Structure Infections Staphylococcus aureus Streptococcus pyogenes
Pediatric Patients
Acute Bacterial Otitis Media Haemophilus influenzae Streptococcus pneumoniae Moraxella catarrhalis
Pharyngitis/Tonsillitis Streptococcus pyogenes CLINICAL STUDIES
NOTE: S. pyogenes S. pyogenes
Uncomplicated Skin and Skin Structure Infections Staphylococcus aureus Streptococcus pyogenes
CEFDINIR CONTRAINDICATIONS
WARNINGS
BEFORE THERAPY WITH CEFDINIR IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFDINIR, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFDINIR IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFDINIR OCCURS, THE DRUG SHOULD BE DISCONTINUED. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficileC. difficile
C. difficileC. difficile
C. difficile C. difficile
PRECAUTIONS
General
DOSAGE AND ADMINISTRATION
Information for Patients
Drug Interactions
Antacids (aluminum- or magnesium-containing)
Concomitant administration of 300 mg cefdinir capsules with 30 mL Maalox® TC suspension reduces the rate (Cmax) and extent (AUC) of absorption by approximately 40%. Time to reach Cmax is also prolonged by 1 hour. There are no significant effects on cefdinir pharmacokinetics if the antacid is administered 2 hours before or 2 hours after cefdinir. If antacids are required during cefdinir therapy, cefdinir should be taken at least 2 hours before or after the antacid.
Probenecid
½
Iron Supplements and Foods Fortified With Iron
4
Drug or Laboratory Test Interactions
®® ®
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitro in vivo 2
Pregnancy
Teratogenic Effects
22
Labor and Delivery
Nursing Mothers
Pediatric Use
Geriatric Use
DOSAGE AND ADMINISTRATION
CEFDINIR ADVERSE REACTIONS
Clinical Trials - Cefdinir Capsules (Adult and Adolescent Patients)
| ADVERSE EVENTS ASSOCIATED WITH CEFDINIR CAPSULES U.S. TRIALS IN ADULT AND ADOLESCENT PATIENTS (N = 3841)a |
||
|---|---|---|
|
a1733 males, 2108 females |
||
| Incidence ≥1% |
Diarrhea | 15% |
| Vaginal moniliasis | 4% of Women | |
| Nausea | 3% | |
| Headache | 2% | |
| Abdominal pain | 1% | |
| Vaginitis | 1% of Women | |
| Incidence <1% but >0.1% |
Rash | 0.9% |
| Dyspepsia | 0.7% | |
| Flatulence | 0.7% | |
| Vomiting | 0.7% | |
| Abnormal stools | 0.3% | |
| Anorexia | 0.3% | |
| Constipation | 0.3% | |
| Dizziness | 0.3% | |
| Dry mouth | 0.3% | |
| Asthenia | 0.2% | |
| Insomnia | 0.2% | |
| Leukorrhea | 0.2% of Women | |
| Moniliasis | 0.2% | |
| Pruritus | 0.2% | |
| Somnolence | 0.2% | |
| LABORATORY VALUE CHANGES OBSERVED WITH CEFDINIR CAPSULES U.S. TRIALS IN ADULT AND ADOLESCENT PATIENTS (N = 3841) |
||
|---|---|---|
| a N <3841 for these parameters | ||
| Incidence ≥1% |
↑Urine leukocytes | 2% |
| ↑Urine protein | 2% | |
| ↑Gamma-glutamyltransferasea | 1% | |
| ↓Lymphocytes, ↑Lymphocytes | 1%, 0.2% | |
| ↑Microhematuria | 1% | |
| Incidence <1% but >0.1% |
↑Glucosea | 0.9% |
| ↑Urine glucose | 0.9% | |
| ↑White blood cells, ↓White blood cells | 0.9%, 0.7% | |
| ↑Alanine aminotransferase (ALT) | 0.7% | |
| ↑Eosinophils | 0.7% | |
| ↑Urine specific gravity, ↓Urine specific gravitya | 0.6%, 0.2% | |
| ↓Bicarbonatea | 0.6% | |
| ↑Phosphorus, ↓Phosphorusa | 0.6%, 0.3% | |
| ↑Aspartate aminotransferase (AST) | 0.4% | |
| ↑Alkaline phosphatase | 0.3% | |
| ↑Blood urea nitrogen (BUN) | 0.3% | |
| ↓Hemoglobin | 0.3% | |
| ↑Polymorphonuclear neutrophils (PMNs), ↓PMNs | 0.3%, 0.2% | |
| ↑Bilirubin | 0.2% | |
| ↑Lactate dehydrogenasea | 0.2% | |
| ↑Platelets | 0.2% | |
| ↑Potassiuma | 0.2% | |
| ↑Urine pHa | 0.2% | |
Clinical Trials - Cefdinir for Oral Suspension (Pediatric Patients)
| ADVERSE EVENTS ASSOCIATED WITH CEFDINIR SUSPENSION U.S. TRIALS IN PEDIATRIC PATIENTS (N = 1783)a |
||
|---|---|---|
|
a 977 males, 806 females b Laboratory changes were occasionally reported as adverse events. |
||
| Incidence ≥ 1% |
Diarrhea |
8% |
| Rash |
3% |
|
| Vomiting |
1% |
|
| Incidence <1% but >0.1% |
Cutaneous moniliasis |
0.9% |
| Abdominal pain |
0.8% |
|
| Leukopeniab
|
0.3% |
|
| Vaginal moniliasis |
0.3% of girls |
|
| Vaginitis |
0.3% of girls |
|
| Abnormal stools |
0.2% |
|
| Dyspepsia |
0.2% |
|
| Hyperkinesia |
0.2% |
|
| Increased ASTb
|
0.2% |
|
| Maculopapular rash |
0.2% |
|
| Nausea |
0.2% |
|
| LABORATORY VALUE CHANGES OF POSSIBLE CLINICAL SIGNIFICANCE OBSERVED WITH CEFDINIR SUSPENSION U.S. TRIALS IN PEDIATRIC PATIENTS (N = 1783) |
||
|---|---|---|
|
a N = 1387 for these parameters
|
||
| Incidence ≥1% |
↑Lymphocytes, ↓Lymphocytes |
2%, 0.8% |
| ↑Alkaline phosphatase |
1% |
|
| ↓Bicarbonatea
|
1% |
|
| ↑Eosinophils |
1% |
|
| ↑Lactate dehydrogenase |
1% |
|
| ↑Platelets |
1% |
|
| ↑PMNs, ↓PMNs |
1%, 1% |
|
| ↑Urine protein |
1% |
|
| Incidence <1% but >0.1% |
↑Phosphorus, ↓Phosphorus |
0.9%, 0.4% |
| ↑Urine pH |
0.8% |
|
| ↓White blood cells, ↑White blood cells |
0.7%, 0.3% |
|
| ↓Calciuma
|
0.5% |
|
| ↓Hemoglobin |
0.5% |
|
| ↑Urine leukocytes |
0.5% |
|
| ↑Monocytes |
0.4% |
|
| ↑AST |
0.3% |
|
| ↑Potassiuma
|
0.3% |
|
| ↑Urine specific gravity, ↓Urine specific gravity |
0.3%, 0.1% |
|
| ↓Hematocrita
|
0.2% |
|
Postmarketing Experience
Cephalosporin Class Adverse Events
WARNINGS
DOSAGE AND ADMINISTRATIONOVERDOSAGE
OVERDOSAGE
CEFDINIR DOSAGE AND ADMINISTRATION
INDICATIONS AND USAGE
| Type of Infection | Dosage | Duration |
|---|---|---|
| Community-Acquired Pneumonia |
300 mg q12h |
10 days |
| Acute Exacerbations of Chronic Bronchitis |
300 mg q12h or 600 mg q24h |
5 to 10 days 10 days |
| Acute Maxillary Sinusitis |
300 mg q12h or 600 mg q24h |
10 days 10 days |
| Pharyngitis/Tonsillitis |
300 mg q12h or 600 mg q24h |
5 to 10 days 10 days |
| Uncomplicated Skin and Skin Structure Infections |
300 mg q12h |
10 days |
Patients With Renal Insufficiency
cr
cr (weight) (140 – age)
cr
(3)
cr body length or height
(4)(5)
2
2
Patients on Hemodialysis
HOW SUPPLIED
Cefdinir Capsules, USP 300 mg
Store at
CLINICAL STUDIES
Community-Acquired Bacterial Pneumonia
| Cefdinir BID | Cefaclor TID | Outcome | |
|---|---|---|---|
|
Clinical Cure Rates
|
150/187 (80%) |
147/186 (79%) |
Cefdinir equivalent to control |
|
Eradication Rates
|
|||
| Overall |
177/195 (91%) |
184/200 (92%) |
Cefdinir equivalent to control |
|
S. pneumoniae
|
31/31 (100%) |
35/35 (100%) |
|
|
H. influenzae
|
55/65 (85%) |
60/72 (83%) |
|
|
M. catarrhalis
|
10/10 (100%) |
11/11 (100%) |
|
|
H. parainfluenzae
|
81/89 (91%) |
78/82 (95%) |
| Cefdinir BID | Amoxicillin/Clavulanate TID |
Outcome | |
|---|---|---|---|
|
Clinical Cure Rates
|
83/104 (80%) |
86/97(89%) |
Cefdinir not equivalent to control |
|
Eradication Rates
|
|||
| Overall |
85/96 (89%) |
84/90 (93%) |
Cefdinir equivalent to control |
|
S. pneumoniae
|
42/44 (95%) |
43/44 (98%) |
|
|
H. influenzae
|
26/35 (74%) |
21/26 (81%) |
|
|
M. catarrhalis
|
6/6 (100%) |
8/8 (100%) |
|
|
H. parainfluenzae
|
11/11 (100%) |
12/12 (100%) |
Streptococcal Pharyngitis or Tonsillitis
| Study | Efficacy Parameter | Cefdinir QD | Cefdinir BID | Penicillin QID | Outcome |
|---|---|---|---|---|---|
| Adults/Adolescents |
Eradication of S. pyogenes
|
192/210 (91%) |
199/217 (92%) |
181/217 (83%) |
Cefdinir superior to control |
| Clinical Cure Rates |
199/210 (95%) |
209/217 (96%) |
193/217 (89%) |
Cefdinir superior to control |
|
| Pediatric Patients |
Eradication of S. pyogenes
|
215/228 (94%) |
214/227 (94%) |
159/227 (70%) |
Cefdinir superior to control |
| Clinical Cure Rates |
222/228 (97%) |
218/227 (96%) |
196/227 (86%) |
Cefdinir superior to control |
| Study | Efficacy Parameter | Cefdinir BID | Penicillin QID | Outcome |
|---|---|---|---|---|
| Adults/Adolescents |
Eradication of S. pyogenes
|
193/218 (89%) |
176/214 (82%) |
Cefdinir equivalent to control |
| Clinical Cure Rates |
194/218 (89%) |
181/214 (85%) |
Cefdinir equivalent to control |
|
| Pediatric Patients |
Eradication of S. pyogenes
|
176/196 (90%) |
135/193 (70%) |
Cefdinir superior to control |
| Clinical Cure Rates |
179/196 (91%) |
173/193 (90%) |
Cefdinir equivalent to control |
REFERENCES
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 4th ed. Approved Standard, NCCLS Document M7-A4, Vol 17(2) NCCLS, Villanova, PA, Jan 1997.
- National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disk Susceptibility Tests, 6th ed. Approved Standard, NCCLS Document M2-A6, Vol 17(1). NCCLS, Villanova, PA, Jan 1997.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine, Nephron 1976;16:31-41.
- Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976;58:259-63.
- Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatrics 1984;104:849-54.
Maalox® TC is a registered trademark of Novartis Consumer Health, Inc.
Clinitest® is a registered trademark of Miles, Inc.
Clinistix® is a registered trademark of Bayer Corporation.
Tes-Tape® is a registered trademark of Lilly Inc.
Manufactured for: Northstar Rx LLC
Memphis, TN 38141
Toll Free: 1-800-206-7821
Issued: 01/2013
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (30 Capsule Bottle)
Rx only
NDC 16714-391-01
Cefdinir Capsules, USP
300 mg
30 Capsules
Northstar Rx LLC

CefdinirCefdinir CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||