Cefaclor
CEFACLOR EXTENDED-RELEASE TABLETS USP, 500 mg1087Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- CEFACLOR DESCRIPTION
- CLINICAL PHARMACOLOGY
- CEFACLOR INDICATIONS AND USAGE
- CEFACLOR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEFACLOR ADVERSE REACTIONS
- OVERDOSAGE
- CEFACLOR DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- REFERENCES
FULL PRESCRIBING INFORMATION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefaclor extended-release tablets USP and other antibacterial drugs, cefaclor extended-release tablets USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
CEFACLOR DESCRIPTION
Cefaclor, USP, the active ingredient in cefaclor extended-release tablets USP, is a semisynthetic cephalosporin antibiotic for oral administration. Cefaclor, USP, is chemically designated as 3-chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate. The cefaclor extended-release tablets formulation of cefaclor differs pharmacokinetically from the immediate-release formulation of cefaclor.
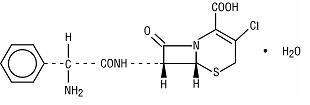
C15H14ClN3O4S • H2O M.W. 385.82
Each cefaclor extended-release tablet contains cefaclor monohydrate equivalent to 500 mg (1.36 mmol) anhydrous cefaclor. In addition, each extended-release tablet contains the following inactive ingredients: FD&C Blue #2 - indigo carmine lake, hypromellose, magnesium stearate, mannitol, polyethylene glycol, povidone and titanium dioxide.
CLINICAL PHARMACOLOGY
Pharmacokinetics
The cefaclor extended-release tablet formulation of cefaclor is pharmacokinetically different from the cefaclor immediate-release capsule formulation of cefaclor. (See TABLE 1.) No direct comparisons with the suspension formulation of cefaclor have been conducted; therefore, there are no data with which to compare the pharmacokinetic properties of the extended-release tablet formulation and the suspension formulation. Until further data are available, the pharmacokinetic equivalence of the extended-release tablet and the suspension formulations should NOT be assumed.
Absorption and Metabolism
The extent of absorption (AUC) and the maximum plasma concentration (Cmax) of cefaclor from cefaclor extended-release tablets are greater when the extended-release tablet is taken with food.
[NOTE: The extent of absorption (AUC) of cefaclor from cefaclor immediate-release capsules is unaffected by food intake; however, when cefaclor immediate-release capsules are taken with food, the Cmax is decreased.]
There is no evidence of metabolism of cefaclor in humans.
Comparative Serum Pharmacokinetics
Serum pharmacokinetic parameters for cefaclor extended-release tablets and cefaclor immediate-release capsules are shown in the table below.
| Parameter | Cefaclor Extended-Release Tablets | Cefaclor Extended-Release Tablets | Cefaclor Immediate-Release Capsules | |||
| 375 mg | 500 mg | 2 x 250 mg | ||||
| fed | fast | fed | fast | fed | fast | |
| n = 10 | n = 16 | n = 16 | n = 15 | n = 16 | ||
| Cmax | 3.7 (1.1) | NA | 8.2 (4.2) | 5.4 (1.6) | 9.3 (2.7) | 16.8 (4.7) |
| Tmax | 2.7 (1.0) | NA | 2.5 (0.8) | 1.5 (0.7) | 1.5 (0.6) | 0.9 (0.4) |
| AUC | 9.9 (2.2) | NA | 18.1 (4.2) | 14.8 (4.0) | 20.5 (2.8) | 19.2 (5.0) |
| (± 1 standard deviation) | ||||||
| NA = data not available | ||||||
No drug accumulation was noted when cefaclor extended-release tablets were given twice daily.
The plasma half-life in healthy subjects is independent of dosage form and averages approximately 1 hour.
Food Effect on Pharmacokinetics
When cefaclor extended-release tablets are taken with food, the AUC is 10% lower while the Cmax is 12% lower and occurs 1 hour later compared to cefaclor immediate-release capsules. In contrast, when cefaclor extended-release tablets are taken without food, the AUC is 23% lower while the Cmax is 67% lower and occurs 0.6 hours later, using an equivalent milligram dose of cefaclor immediate-release capsules as a reference. Therefore, cefaclor extended-release tablets should be taken with food.
Special Populations
Renal Insufficiency
In patients with reduced renal function, the serum half-life of cefaclor is slightly prolonged. In those with complete absence of renal function, the plasma half-life of the intact molecule is 2.3 to 2.8 hours. Excretion pathways in patients with markedly impaired renal function have not been determined. Hemodialysis shortens the half-life by 25% to 30%.
Geriatric Patients
In elderly subjects (over age 65) with normal serum creatinine values, higher peak plasma concentrations and AUCs have been observed. This is considered to be primarily a result of an age-related decrement in renal function, and has no apparent clinical significance. Therefore, dosage adjustment is not necessary in elderly subjects with normal serum creatinine values.
Microbiology
Cefaclor has in vitro activity against a broad range of gram-positive and gram-negative bacteria. The bactericidal action of cefaclor results from inhibition of cell-wall synthesis. Cefaclor is stable in the presence of some bacterial ß-lactamases; consequently, some ß-lactamase-producing organisms may be susceptible to cefaclor.
Cefaclor extended-release tablets have been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Gram-positive aerobes:
Staphylococcus aureus
Streptococcus pneumoniae
Streptococcus pyogenes
NOTE: Cefaclor is inactive against methicillin-resistant staphylococci.
Gram-negative aerobes:
Haemophilus influenzae (non-ß-lactamase-producing strains only)
Moraxella catarrhalis (including ß-lactamase-producing strains)
The following in vitro data are available, but their clinical significance is unknown . Cefaclor exhibits in vitro minimum inhibitory concentrations (MICs) of 8 mcg/mL or less (systemic susceptibility breakpoint) against most (≥ 90%) strains of the following microorganisms; however, the safety and effectiveness of cefaclor extended-release tablets in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.
Gram-positive aerobes:
Staphylococcus epidermidis
Gram-negative aerobes:
Haemophilus parainfluenzae
Klebsiella pneumoniae
Anaerobic bacteria:
Peptococcus niger
Peptostreptococci
Propionibacterium acnes
NOTE: Acinetobacter calcoaceticus, Enterobacter spp., Entercoccus spp., Morganella morganii, Proteus vulgaris, Providencia spp., Pseudomonas spp., and Serratia spp. are resistant to cefaclor.
Susceptibility Testing
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth, agar, or microdilution) or equivalent with standardized inoculum concentrations and standardized amounts of cefaclor powder. The MIC values should be interpreted according to the following criteria:
| MIC (mcg/mL) | Interpretation |
| ≤ 8 | Susceptible (S) |
| 16 | Intermediate (I) |
| ≥ 32 | Resistant (R) |
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard cefaclor powder should provide the following MIC values:
| Microorganism | MIC range (mcg/mL) | |
| E. coli | ATCC 25922 | 1 to 4 |
| E. faecalis | ATCC 29212 | > 32 |
| S. aureus | ATCC 29213 | 1 to 4 |
| H. influenzae | ATCC 49766 |
1 to 4 |
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 mcg cefaclor to test the susceptibility of microorganisms to cefaclor.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 mcg cefaclor disk should be interpreted according to the following criteria:
| Zone diameter (mm) | Interpretation |
| ≥ 18 | Susceptible (S) |
| 15 to 17 | Intermediate (I) |
| ≤ 14 | Resistant (R) |
When testing* H. Influenzae, the following interpretive criteria should be used:
| Zone diameter (mm) | Interpretation |
| ≥ 20 | Susceptible (S) |
| 17 to 19 | Intermediate (I) |
| ≤ 16 | Resistant (R) |
* Disk susceptibility tests performed using Haemophilus Test Medium (HTM)2
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cefaclor.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30 mcg cefaclor disk should provide the following zone diameters in these laboratory test quality control strains:
| Microorganism | Zone diameter (mm) | |
| E. coli | ATCC 25922 | 23 to 27 |
| S. aureus | ATCC 25923 | 27 to 31 |
|
H. influenzae
|
ATCC 49766 | 25 to 31 |
CEFACLOR INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefaclor extended-release tablets USP and other antibacterial drugs, cefaclor extended-release tablets USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
The safety and effectiveness of cefaclor extended-release tablets in treating some of the indications and pathogens for which other formulations of cefaclor are approved have NOT been established. When administered at the recommended dosages and durations of therapy, cefaclor extended-release tablets are indicated for the treatment of patients with the following mild to moderate infections when caused by susceptible strains of the designated organisms. (See DOSAGE AND ADMINISTRATION and CLINICAL STUDIES sections.)
Acute bacterial exacerbations of chronic bronchitis due to Haemophilus influenzae (non-ß-lactamase-producing strains only), Moraxella catarrhalis (including ß-lactamase-producing strains) or Streptococcus pneumoniae.
NOTE: In view of the insufficient numbers of isolates of ß-lactamase-producing strains of Haemophilus influenzae that were obtained from clinical trials with cefaclor extended-release tablets for patients with acute bacterial exacerbations of chronic bronchitis or secondary bacterial infections of acute bronchitis, it was not possible to adequately evaluate the effectiveness of cefaclor extended-release tablets for bronchitis known, suspected, or considered potentially to be caused by ß-lactamase-producing H. influenzae.
Secondary bacterial infections of acute bronchitis due to Haemophilus influenzae (non-ß-lactamase-producing strains only), Moraxella catarrhalis (including ß-lactamase-producing strains), or Streptococcus pneumoniae. (See above NOTE.)
Pharyngitis and tonsillitis due to Streptococcus pyogenes.
NOTE: Only penicillin by the intramuscular route of administration has been shown to be effective in the prophylaxis of rheumatic fever. Cefaclor extended-release tablets are generally effective in the eradication of S. pyogenes from the oropharynx; however, data establishing the efficacy of cefaclor extended-release tablets for the prophylaxis of subsequent rheumatic fever are not available.
Uncomplicated skin and skin and structure infections due to Staphylococcus aureus (methicillin-susceptible).
NOTE: In view of the insufficient numbers of isolates of Streptococcus pyogenes that were obtained from clinical trials with cefaclor extended-release tablets for patients with uncomplicated skin and skin structure infections, it was not possible to adequately evaluate the effectiveness of cefaclor extended-release tablets for skin infections known, suspected, or considered potentially to be caused by S. pyogenes.
CEFACLOR CONTRAINDICATIONS
Cefaclor extended-release tablets are contraindicated in patients with known hypersensitivity to cefaclor and other cephalosporins.
WARNINGS
BEFORE THERAPY WITH CEFACLOR EXTENDED-RELEASE TABLETS IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFACLOR, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-SENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFACLOR EXTENDED-RELEASE TABLETS OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefaclor and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increase morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS
General
Prescribing cefaclor extended-release tablets USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Superinfection (overgrowth by non-susceptible organisms) should always be considered a possibility in a patient being treated with a broad spectrum antimicrobial. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Information for Patients
Patients should be counseled that antibacterial drugs including cefaclor extended-release tablets USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefaclor extended-release tablets USP are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefaclor extended-release tablets USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions
Antacids
The extent of absorption of cefaclor extended-release tablets is diminished if magnesium or aluminum hydroxide-containing antacids are taken within 1 hour of administration; H2 blockers do not alter either the rate or the extent of absorption of cefaclor extended-release tablets.
Probenecid
The renal excretion of cefaclor is inhibited by probenecid.
Warfarin
There have been rare reports of increased prothrombin time with or without clinical bleeding in patients receiving cefaclor and warfarin concomitantly. No specific studies have been performed to rule in or rule out this potential drug/drug interaction.
Laboratory Test Interactions
Administration of cefaclor extended-release tablets may result in a false-positive reaction for glucose in the urine. This phenomenon has been seen in patients taking cephalosporin antibiotics when the test is performed using Benedict's and Fehling's solutions and also with Clinitest® tablets.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals have not been performed to evaluate the carcinogenic or mutagenic potential for cefaclor. Reproduction studies have revealed no evidence of impaired fertility.
Usage in Pregnancy
Teratogenic Effect
Pregnancy category B
Reproduction studies using cefaclor have been performed in mice, rats and ferrets at doses up to 3 to 5 times the maximum human dose (1500 mg/day) based on mg/m2. These studies have revealed no harm to the fetus due to cefaclor. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, cefaclor extended-release tablets should be used during pregnancy only if clearly needed.
Labor and Delivery
Cefaclor extended-release tablets have not been studied for use during labor and delivery. Treatment should be given only if clearly needed.
Nursing Mothers
No studies in lactating women have been performed with cefaclor extended-release tablets. Small amounts of cefaclor (≤ 0.21 mcg/mL) have been detected in human milk following administration of single 500 mg doses of cefaclor extended-release tablets. The effect on nursing infants is not known. Caution should be exercised when cefaclor extended-release tablets are administered to a nursing woman.
Pediatric Use
Safety and effectiveness of cefaclor extended-release tablets in pediatric patients less than 16 years of age have not been established.
Geriatric Use
Healthy geriatric volunteers (≥ 65 years old) who received a single 750 mg dose of cefaclor extended-release tablets had 40% to 50% higher AUC and 20% lower renal clearance values when compared to healthy adult volunteers less than 45 years of age. These differences are considered to be primarily a result of age-related decreases in renal function. In clinical studies when geriatric patients received the usual recommended adult doses, clinical efficacy and safety were comparable to results in non-geriatric adult patients. No dosage changes are recommended for healthy geriatric patients.
CEFACLOR ADVERSE REACTIONS
Clinical Trials
There were 3272 patients treated with multiple doses of cefaclor extended-release tablets in controlled clinical trials and an additional 211 subjects in pharmacology studies. There were no deaths in these trials thought to be related to toxicity from cefaclor extended-release tablets. Treatment was discontinued in 1.7% of patients due to adverse events thought to be possibly or probably drug-related.
The following adverse clinical and laboratory events were reported during the cefaclor extended-release tablets clinical trials conducted in North America at doses of 375 mg or 500 mg BID; however, relatedness of the adverse events to the drug was not assigned by clinical investigators during the trials (see TABLES 2 and 3).
| Incidence Equal to or Greater Than 1% | Event | Incidence |
| Headache | 4.9% | |
| Rhinitis | 3.9% | |
| Diarrhea | 3.8% | |
| Nausea | 3.4% | |
Vaginitis |
2.4% | |
Vaginal Moniliasis |
2.2% | |
| Abdominal Pain | 1.6% | |
| Cough Increased | 1.5% | |
| Pharyngitis | 1.4% | |
| Pruritus | 1.4% | |
| Back Pain | 1.0% |
Adverse reactions occurring during the clinical trials with cefaclor extended-release tablets with an incidence of less than 1% but greater than 0.1% included the following (listed alphabetically):
Accidental injury, anorexia, anxiety, arthralgia, asthma, bronchitis, chest pain, chills, congestive heart failure, conjunctivitis, constipation, dizziness, dysmenorrhea, dyspepsia, dysuria, ear pain, edema, fever, flatulence, flu syndrome, gastritis, infection, insomnia, leukorrhea, lung disorder, maculopapular rash, malaise, menstrual disorder, myalgia, nausea and vomiting, neck pain, nervousness, nocturia, otitis media, pain, palpitation, peripheral edema, rash, respiratory disorder, sinusitis, somnolence, surgical procedure, sweating, tremor, urticaria, vomiting.
NOTE: One case of serum-sickness-like reaction was reported among the 3272 adult patients treated with cefaclor extended-release tablets during the controlled clinical trials. These reactions have also been reported with the use of cefaclor in other oral formulations and are seen more frequently in pediatric patients than in adults. These reactions are characterized by findings of erythema multiforme, rash, and other skin manifestations accompanied by arthritis/arthralgia, with or without fever, and differ from classic serum sickness in that there is infrequently associated lymphadenopathy and proteinuria, no circulating immune complexes and no evidence to date of sequelae of the reaction. While further investigation is ongoing, serum-sickness-like reactions appear to be due to hypersensitivity and more often occur during or following a second (or subsequent) course of therapy with cefaclor. Such reactions have been reported with overall occurrence ranging from 1 in 200 (0.5%) in one focused trial; to 2 in 8346 (0.024%) in overall clinical trials (with an incidence in pediatric patients in clinical trials of 0.055%); to 1 in 38,000 (0.003%) in spontaneous event reports. Signs and symptoms usually occur a few days after initiation of therapy and subside within a few days after cessation of therapy. Occasionally these reactions have resulted in hospitalization, usually of short duration (median hospitalization = 2 to 3 days, based on postmarketing surveillance studies). In those patients requiring hospitalization, the symptoms have ranged from mild to severe at the time of admission with more of the severe reactions occurring in pediatric patients.
| Event | Incidence | |
| Incidence Less Than 1%, but Greater Than 0.1% | Albumin decreased | 0.3% |
| Alkaline phosphatase increased | 0.3% | |
| ALT/SGPT increased | 0.3% | |
| Bilirubin total increased | 0.3% | |
| Blood urea nitrogen (BUN) increased | 0.2% | |
| Calcium decreased | 0.7% | |
| Creatine phosphokinase increased | 0.7% | |
| Creatinine increased | 0.5% | |
| Eosinophils increased | 0.3% | |
| Erythrocyte count decreased | 0.3% | |
| GGT increased | 0.2% | |
| Hemoglobin decreased | 0.2% | |
| Lymphocytes decreased | 0.3% | |
| Mean Cell Volume (MCV) increased | 0.7% | |
| Neutrophils segmented decreased | 0.3% | |
| Phosphorous increased | 0.7% | |
| Platelet count decreased | 0.3% | |
| Potassium increased | 0.4% | |
| Sodium decreased | 0.3% | |
| Sodium increased | 0.4% |
In Postmarketing Experience
In addition to the events reported during clinical trials with cefaclor extended-release tablets, the following adverse experiences are among those that have been reported during worldwide postmarketing surveillance: allergic reaction, anaphylactoid reaction, angioedema, face edema, hypotension, Stevens-Johnson syndrome, syncope, paresthesia, vasodilatation and vertigo.
Other Side Effects Associated With Other Formulations of Cefaclor
In addition to the above, the following other adverse reactions and altered laboratory tests have been associated with cefaclor in other oral formulations:
Clinical
Severe hypersensitivity reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and anaphylaxis, have been reported rarely. Anaphylactoid events may be manifested by solitary symptoms, including angioedema, edema (including face and limbs), paresthesias, syncope, or vasodilatation. Anaphylaxis may be more common in patients with a history of penicillin allergy. Rarely, hypersensitivity symptoms may persist for several months.
Symptoms of pseudomembranous colitis may appear either during or after antibiotic treatment. (See WARNINGS.)
Laboratory
Abnormal urinalysis, eosinophilia, leukopenia, neutropenia, transient elevations in AST, and transient thrombocytopenia have been reported.
Cephalosporin-Class Reactions
In addition to the adverse reactions listed above, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Clinical
Confusion, erythema multiforme, genital pruritus, hepatic dysfunction including cholestasis, hemolytic anemia, reversible hyperactivity, hypertonia, and reversible interstitial nephritis.
Laboratory
Positive direct Coombs’ test.
OVERDOSAGE
The toxic symptoms following an overdose of cefaclor may include nausea, vomiting, epigastric distress, and diarrhea. The severity of the epigastric distress and the diarrhea are dose-related.
Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage. Consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed.
Although cefaclor is considered dialyzable, neither forced diuresis, peritoneal dialysis, hemodialysis, nor charcoal hemoperfusion have been demonstrated to be beneficial in an overdose of cefaclor.
CEFACLOR DOSAGE AND ADMINISTRATION
The absorption of cefaclor extended-release tablets is enhanced when it is administered with food. (See CLINICAL PHARMACOLOGY.) Therefore, cefaclor extended-release tablets should be administered with meals (i.e., at least within one hour of eating). The extended-release tablets should not be cut, crushed, or chewed.
See INDICATIONS AND USAGE for information about patients for whom cefaclor extended-release tablets are indicated.
NOTE: 500 mg BID of cefaclor extended-release tablets is clinically equivalent to 250 mg TID of cefaclor immediate-release as a capsule in those indications listed in the INDICATIONS AND USAGE section of this label. 500 mg BID of cefaclor extended-release tablets is NOT equivalent to 500 mg TID of other cefaclor formulations.
Adults (age 16 years and older):
| Type of Infection (as qualified in the INDICATIONS AND USAGE section of this labeling) | Total Daily Dose | Dose and Frequency | Duration |
| Acute Bacterial Exacerbations of Chronic Bronchitis due to H. influenzae (non-ß-lactamase-producing strains only). Moraxella catarrhalis (including ß-lactamase-producing strains), or Streptococcus pneumoniae (See INDICATIONS AND USAGE.) | 1000 mg | 500 mg q 12 hours | 7 days |
| Secondary Bacterial Infection of Acute Bronchitis due to H. influenzae (non-ß-lactamase-producing strains only). M. catarrhalis (including ß-lactamase-producing strains), or S. pneumoniae (See INDICATIONS AND USAGE.) | 1000 mg | 500 mg q 12 hours | 7 days |
| Pharyngitis and/or tonsillitis due to S. pyogenes | 750 mg | 375 mg q 12 hours | 10 days |
| Uncomplicated Skin and Skin Structure Infections due to S. aureus (methicillin-susceptible strains) (See INDICATIONS AND USAGE.) | 750 mg | 375 mg q 12 hours | 7 to 10 days |
Elderly patients with normal renal function do not require dosage adjustments.
HOW SUPPLIED
Cefaclor extended-release tablets USP, 500 mg (based on the anhydrous), are available as film-coated, oval-shaped, unscored, dark blue tablets, debossed with “93” on one side and “1087” on the other side. They are available in bottles of 100.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
CLINICAL STUDIES
ACUTE BACTERIAL EXACERBATIONS OF CHRONIC BRONCHITIS AND SECONDARY BACTERIAL INFECTIONS OF ACUTE BRONCHITIS
In adequate and well-controlled clinical trials of cefaclor extended-release tablets in the treatment of acute bacterial exacerbations of chronic bronchitis (ABECB) and secondary bacterial infections of acute bronchitis (SBIAB), only 4 evaluable patients with ABECB and no evaluable patients with SBIAB had infections caused by ß-lactamase-producing H. influenzae. Four patients do not provide adequate data upon which to judge clinical efficacy of cefaclor extended-release tablets against ß-lactamase-producing H. influenzae.
UNCOMPLICATED SKIN AND SKIN STRUCTURE INFECTIONS
Cefaclor extended-release tablets (375 mg Q12H) (n = 115) were compared to cefaclor immediate-release capsules (250 mg TID) (n = 106) for the treatment of patients with uncomplicated skin and skin structure infections, including cellulitis, pyoderma, abscess and impetigo. Patients were treated for 7 to 10 days and were evaluated for clinical resolution and bacterial eradication approximately one week after completing therapy. To be evaluable, all patients had to have a recognized pathogen isolated from the skin infection just prior to the initiation of therapy. The results of this randomized, double-blinded, U.S. trial demonstrated:
- overall clinical cure rates were 72% (83 of 115 patients) and 75% (80 of 106 patients), respectively, for cefaclor extended-release tablets and cefaclor immediate-release capsules [95% CI around the 3% difference = -16% to +9%],
- overall bacteriologic eradication rates against Staphylococcus aureus were comparable (see TABLE 4).
| Outcome by Pathogen | CEFACLOR EXTENDED-RELEASE TABLETS | CEFACLOR IMMEDIATE-RELEASE CAPSULES | ||
| Staphylococcus aureus | 67/95 | (71%) | 58/81 | (71%) |
| Streptococcus pyogenes | 10/16 | (63%) | 8/9 | (89%) |
| Other streptococci | 7/11 | (64%) | 5/6 | (83%) |
| Total | 84/122 | (69%) | 71/96 | (74%) |
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically—Third edition; Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA, December 1993.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests—Fifth edition; Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, December 1993.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Teva Pharmaceuticals USA.
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. J 6/2010

Cefaclor Extended-Release Tablets 500 mg 100s Label Text
NDC 0093-1087-01
CEFACLOR
EXTENDED-RELEASE
Tablets USP
500 mg
Each tablet contains:
Cefaclor, USP, equivalent
to 500 mg of anhydrous cefaclor.
Rx only
100 Tablets
TEVA
CefaclorCefaclor TABLET, FILM COATED, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||