Carbamazepine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- CARBAMAZEPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- CARBAMAZEPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- CARBAMAZEPINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
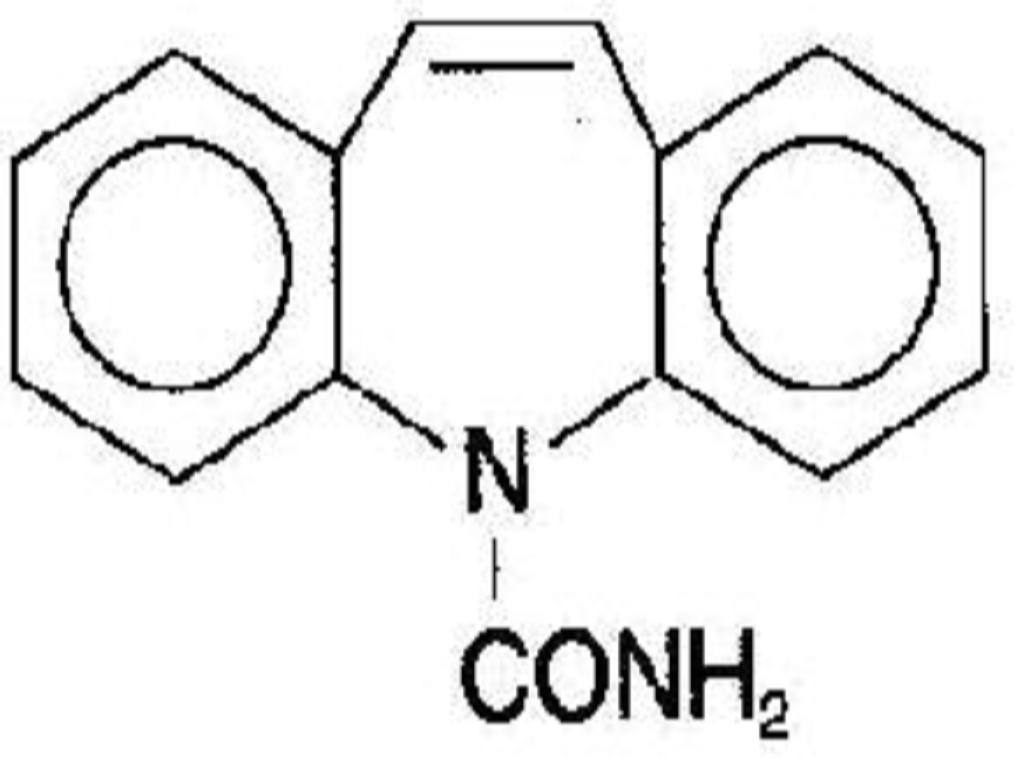
CARBAMAZEPINE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of Action
PHARMACOKINETICS
Drug InteractionsINDICATIONS & USAGE
EpilepsyGeneral
Trigeminal Neuralgia
CARBAMAZEPINE CONTRAINDICATIONS
WARNINGS
Serious Dermatologic ReactionsSJS/TEN and HLA-B*1502 Allele
WARNINGSLaboratory Tests
Aplastic Anemia and Agranulocytosis
Suicidal Behavior and Ideation
General
Usage in Pregnancy
PRECAUTIONS
GeneralINDICATIONS AND USAGE
ADVERSE REACTIONSLaboratory Tests
ADVERSE REACTIONS, OtherInformation for Patients
INFORMATION FOR PATIENTS
Usage in Pregnancy
LABORATORY TESTS
WARNINGSGeneralADVERSE REACTIONS
CLINICAL PHARMACOLOGY
Drug Interactions
Agents That May Affect Carbamazepine Plasma Levels
Effect of Carbamazepine on Plasma Levels of Concomitant Agents
CONTRAINDICATIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGSLABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
INDICATIONS AND USAGEGERIATRIC USE
CARBAMAZEPINE ADVERSE REACTIONS
BOXED WARNING
BOXED WARNING
Laboratory Tests
GeneralInformation for Patients
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
Signs and Symptoms
Treatment
DOSAGE AND ADMINISTRATION (SEE TABLE BELOW)
Laboratory Tests
INDICATIONS AND USAGE
Drug InteractionsPregnancy Category D
INDICATIONS AND USAGE
Dosage Information
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


CarbamazepineCarbamazepine TABLET, CHEWABLE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!