BUPROBAN
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BUPROBAN safely and effectively. See full prescribing information for BUPROBAN. BUPROBAN [buPROPion HCl Extended-Release Tablets (SR)], for oral useInitial U.S. Approval: 1985BOXED WARNINGWARNING: NEUROPSYCHIATRIC REACTIONS; AND SUICIDAL THOUGHTS AND BEHAVIORS See full prescribing information for complete boxed warning. Serious neuropsychiatric events have been reported in patients taking bupropion for smoking cessation. (5.1) Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants (5.2) Monitor for worsening and emergence of suicidal thoughts and behaviors. (5.2) RECENT MAJOR CHANGES Dosage and Administration (2.8) 03/2014 Contraindications (4) 03/2014 INDICATIONS AND USAGEBUPROBAN® is an aminoketone agent indicated as an aid to smoking cessation treatment. (1)DOSAGE AND ADMINISTRATION Starting dose: 150 mg per day for first 3 days. (2.1) General: Increase dose gradually to reduce seizure risk. (2.1, 5.3) Begin dosing one week before quit day. (2.1) After 3 days, increase the dose to 300 mg per day, given as 150 mg twice daily at an interval of at least 8 hours. (2.1) May be used with a nicotine transdermal system. (2.5) Moderate to severe hepatic impairment: 150 mg every other day. (2.6, 8.7) Mild hepatic impairment: Consider reducing the dose and/or frequency of dosing. (2.6, 8.7) Renal Impairment: Consider reducing the dose and/or frequency. (2.7, 8.6) DOSAGE FORMS AND STRENGTHS Tablets: 150 mg. (3) CONTRAINDICATIONS Seizure disorder. (4, 5.3) Current or prior diagnosis of bulimia or anorexia nervosa. (4, 5.3) Abrupt discontinuation of alcohol, benzodiazepines, barbiturates, antiepileptic drugs. (4, 5.3) Monoamine Oxidase Inhibitors (MAOIs): Do not use MAOIs intended to treat psychiatric disorders with BUPROBAN® or within 14 days of stopping treatment with BUPROBAN®. Do not use BUPROBAN® within 14 days of stopping an MAOI intended to treat psychiatric disorders. In addition, do not start BUPROBAN® in a patient who is being treated with linezolid or intravenous methylene blue. (4, 7.6) Known hypersensitivity to bupropion or other ingredients of BUPROBAN®. (4, 5.7) WARNINGS AND PRECAUTIONS Seizure risk: The risk is dose-related. Can minimize risk by gradually increasing the dose and limiting daily dose to 300 mg. Discontinue if seizure occurs. (4, 5.3, 7.3) Hypertension: BUPROBAN® can increase blood pressure. Monitor blood pressure before initiating treatment and periodically during treatment, especially if used with nicotine replacement. (5.4) Activation of mania/hypomania: Screen patients for bipolar disorder and monitor for these symptoms. (5.5) Psychosis and other neuropsychiatric reactions. Instruct patients to contact a healthcare professional if reactions occur. (5.6) Side EffectsMost common adverse reactions (incidence ≥5% and ≥1% more than placebo rate) are: insomnia, rhinitis, dry mouth, dizziness, nervous disturbance, anxiety, nausea, constipation, and arthralgia. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact TEVA USA, PHARMACOVIGILANCE at 1-866-832-8537 or drug.safety@tevapharm.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS CYP2B6 inducers: Dose increase may be necessary if coadministered with CYP2B6 inducers (e.g., ritonavir, lopinavir, efavirenz, carbamazepine, phenobarbital and phenytoin) based on clinical response, but should not exceed the maximum recommended dose. (7.1) Drugs metabolized by CYP2D6: Bupropion inhibits CYP2D6 and can increase concentrations of: antidepressants (e.g., venlafaxine, nortriptyline, imipramine, desipramine, paroxetine, fluoxetine, sertraline), antipsychotics (e.g., haloperidol, risperidone, thioridazine), beta-blockers (e.g., metoprolol), and Type 1C antiarrhythmics (e.g., propafenone, flecainide). Consider dose reduction when using with bupropion. (7.2) Drugs that lower seizure threshold: Dose BUPROBAN® with caution. (5.3, 7.3) Dopaminergic drugs (levodopa and amantadine): CNS toxicity can occur when used concomitantly with BUPROBAN®. (7.4) MAOIs: Increased risk of hypertensive reactions can occur when used concomitantly with BUPROBAN®. (7.6) Drug-laboratory test interactions: BUPROBAN® can cause false-positive urine test results for amphetamines. (7.8) USE IN SPECIFIC POPULATIONSPregnancy: Use only if benefit outweighs potential risk to the fetus. (8.1)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 BUPROBAN INDICATIONS AND USAGE
- 2 BUPROBAN DOSAGE AND ADMINISTRATION
- 2.1 Usual Dosage
- 2.2 Duration of Treatment
- 2.3 Individualization of Therapy
- 2.4 Maintenance
- 2.5 Combination Treatment With BUPROBAN and a Nicotine Transdermal System (NTS)
- 2.6 Dose Adjustment in Patients With Hepatic Impairment
- 2.7 Dose Adjustment in Patients With Renal Impairment
- 2.8 Use of BUPROBAN With Reversible MAOIs Such as Linezolid or Methylene Blue
- 3 DOSAGE FORMS AND STRENGTHS
- 4 BUPROBAN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 BUPROBAN ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 BUPROBAN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- MEDICATION GUIDE
- PRINCIPAL DISPLAY PANEL - 150 mg Bottle Label
FULL PRESCRIBING INFORMATION
NEUROPSYCHIATRIC REACTIONS IN PATIENTS TAKING BUPROPION FOR SMOKING CESSATION
Serious neuropsychiatric reactions have occurred in patients taking BUPROBAN® for smoking cessation [see Warnings and Precautions (5.1)]. The majority of these reactions occurred during bupropion treatment, but some occurred in the context of discontinuing treatment. In many cases, a causal relationship to bupropion treatment is not certain, because depressed mood may be a symptom of nicotine withdrawal. However, some of the cases occurred in patients taking BUPROBAN® who continued to smoke.
The risks of BUPROBAN® should be weighed against the benefits of its use. BUPROBAN® has been demonstrated to increase the likelihood of abstinence from smoking for as long as 6 months compared with treatment with placebo. The health benefits of quitting smoking are immediate and substantial.
SUICIDALITY AND ANTIDEPRESSANT DRUGS
Although BUPROBAN® is not indicated for treatment of depression, it contains the same active ingredient as the antidepressant medications WELLBUTRIN®, WELLBUTRIN SR®, and WELLBUTRIN XL®. Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term trials. These trials did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in subjects over age 24; there was a reduction in risk with antidepressant use in subjects aged 65 and older [see Warnings and Precautions (5.2)].
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber [see Warnings and Precautions (5.2)].
1 INDICATIONS AND USAGE
BUPROBAN® is indicated as an aid to smoking cessation treatment.
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage
Treatment with BUPROBAN® should be initiated before the patient's planned quit day, while the patient is still smoking, because it takes approximately 1 week of treatment to achieve steady-state blood levels of bupropion. The patient should set a "target quit date" within the first 2 weeks of treatment with BUPROBAN®.
Dosing: To minimize the risk of seizure:
- Begin dosing with one 150-mg tablet per day for 3 days.
- Increase dose to 300 mg/day given as one 150-mg tablet twice each day with an interval of at least 8 hours between each dose.
- Do not exceed 300 mg/day.
BUPROBAN® should be swallowed whole and not crushed, divided, or chewed, as this may lead to an increased risk of adverse effects including seizures [see Warnings and Precautions (5.3)].
BUPROBAN® may be taken with or without food [see Clinical Pharmacology (12.3)].
2.2 Duration of Treatment
Treatment with BUPROBAN® should be continued for 7 to 12 weeks. If the patient has not quit smoking after 7 to 12 weeks, it is unlikely that he or she will quit during that attempt so treatment with BUPROBAN® should probably be discontinued and the treatment plan reassessed. The goal of therapy with BUPROBAN® is complete abstinence.
Discuss discontinuing treatment with BUPROBAN® after 12 weeks if the patient feels ready but consider whether the patient may benefit from ongoing treatment. Patients who successfully quit after 12 weeks of treatment but do not feel ready to discontinue treatment should be considered for ongoing therapy with BUPROBAN®; longer treatment should be guided by the relative benefits and risks for individual patients.
It is important that patients continue to receive counseling and support throughout treatment with BUPROBAN® and for a period of time thereafter.
2.3 Individualization of Therapy
Patients are more likely to quit smoking and remain abstinent if they are seen frequently and receive support from their physicians or other healthcare professionals. It is important to ensure that patients read the instructions provided to them and have their questions answered. Physicians should review the patient's overall smoking cessation program that includes treatment with BUPROBAN®. Patients should be advised of the importance of participating in the behavioral interventions, counseling, and/or support services to be used in conjunction with BUPROBAN® [see Medication Guide].
Patients who fail to quit smoking during an attempt may benefit from interventions to improve their chances for success on subsequent attempts. Patients who are unsuccessful should be evaluated to determine why they failed. A new quit attempt should be encouraged when factors that contributed to failure can be eliminated or reduced, and conditions are more favorable.
2.4 Maintenance
Tobacco dependence is a chronic condition. Some patients may need on-going treatment. Whether to continue treatment with BUPROBAN® for periods longer than 12 weeks for smoking cessation must be determined for individual patients.
2.5 Combination Treatment With BUPROBAN and a Nicotine Transdermal System (NTS)
Combination treatment with BUPROBAN® and NTS may be prescribed for smoking cessation. The prescriber should review the complete prescribing information for both BUPROBAN® and NTS before using combination treatment [see Clinical Studies (14)]. Monitoring for treatment-emergent hypertension in patients treated with the combination of BUPROBAN® and NTS is recommended.
2.6 Dose Adjustment in Patients With Hepatic Impairment
In patients with moderate to severe hepatic impairment (Child-Pugh score: 7 to 15), the maximum dose should not exceed 150 mg every other day. In patients with mild hepatic impairment (Child-Pugh score: 5 to 6), consider reducing the dose and/or frequency of dosing [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.7 Dose Adjustment in Patients With Renal Impairment
Consider reducing the dose and/or frequency of BUPROBAN® in patients with renal impairment (Glomerular Filtration Rate <90 mL/min) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.8 Use of BUPROBAN With Reversible MAOIs Such as Linezolid or Methylene Blue
Do not start BUPROBAN® in a patient who is being treated with a reversible MAOI such as linezolid or intravenous methylene blue. Drug interactions can increase the risk of hypertensive reactions [see Contraindications (4) and Drug Interactions (7.6)]. In some cases, a patient already receiving therapy with BUPROBAN® may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of hypertensive reactions in a particular patient, BUPROBAN® should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with BUPROBAN® may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue.
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with BUPROBAN® is unclear. The clinician should, nevertheless, be aware of the possibility of a drug interaction with such use [see Contraindications (4) and Drug Interactions (7.6)].
3 DOSAGE FORMS AND STRENGTHS
150 mg – light yellow, round, convex, film-coated, extended-release tablets debossed with "G" on one side and "2444" on the other side.
4 CONTRAINDICATIONS
- BUPROBAN® is contraindicated in patients with a seizure disorder.
- BUPROBAN® is contraindicated in patients with a current or prior diagnosis of bulimia or anorexia nervosa as a higher incidence of seizures was observed in such patients treated with the immediate-release formulation of bupropion [see Warnings and Precautions (5.3)].
- BUPROBAN® is contraindicated in patients undergoing abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs [see Warnings and Precautions (5.3) and Drug Interactions (7.3)].
- The use of MAOIs (intended to treat psychiatric disorders) concomitantly with BUPROBAN® or within 14 days of discontinuing treatment with BUPROBAN® is contraindicated. There is an increased risk of hypertensive reactions when BUPROBAN® is used concomitantly with MAOIs. The use of BUPROBAN® within 14 days of discontinuing treatment with an MAOI is also contraindicated. Starting BUPROBAN® in a patient treated with reversible MAOIs such as linezolid or intravenous methylene blue is contraindicated [see Dosage and Administration (2.8), Warnings and Precautions (5.4), and Drug Interactions (7.6)].
- BUPROBAN® is contraindicated in patients with a known hypersensitivity to bupropion or other ingredients of BUPROBAN®. Anaphylactoid/anaphylactic reactions and Stevens-Johnson syndrome have been reported [see Warnings and Precautions (5.7)].
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment
Serious neuropsychiatric symptoms have been reported in patients taking BUPROBAN® for smoking cessation. These have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, hostility, agitation, aggression, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide [see Boxed Warning and Adverse Reactions (6.2)]. Observe patients for the occurrence of neuropsychiatric reactions. Instruct patients to contact a healthcare professional if such reactions occur.
In many of these cases, a causal relationship to bupropion treatment is not certain, because depressed mood can be a symptom of nicotine withdrawal. However, some of the cases occurred in patients taking BUPROBAN® who continued to smoke.
The risks of BUPROBAN® should be weighed against the benefits of its use. BUPROBAN® has been demonstrated to increase the likelihood of abstinence from smoking for as long as 6 months compared with treatment with placebo. The health benefits of quitting smoking are immediate and substantial.
5.2 Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults
Patients with MDD, both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.
Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (selective serotonin reuptake inhibitors [SSRIs] and others) show that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with MDD and other psychiatric disorders. Short-term clinical trials did not show an increase in the risk of suicidality with antidepressants compared with placebo in adults beyond age 24; there was a reduction with antidepressants compared with placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4,400 subjects. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 subjects. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger subjects for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 subjects treated) are provided in Table 1.
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Subjects Treated |
|---|---|
| Increases Compared With Placebo | |
| <18 | 14 additional cases |
| 18 to 24 | 5 additional cases |
| Decreases Compared With Placebo | |
| 25 to 64 | 1 fewer case |
| ≥65 | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases [see Boxed Warning].
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for MDD or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for BUPROBAN® should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
5.3 Seizure
BUPROBAN® can cause seizure. The risk of seizure is dose-related. The dose of BUPROBAN® should not exceed 300 mg per day [see Dosage and Administration (2.1)]. Discontinue BUPROBAN® and do not restart treatment if the patient experiences a seizure.
The risk of seizures is also related to patient factors, clinical situations, and concomitant medications that lower the seizure threshold. Consider these risks before initiating treatment with BUPROBAN®. BUPROBAN® is contraindicated in patients with a seizure disorder, current or prior diagnosis of anorexia nervosa or bulimia, or undergoing abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs [see Contraindications (4) and Drug Interactions (7.3)]. The following conditions can also increase the risk of seizure: severe head injury; arteriovenous malformation; CNS tumor or CNS infection; severe stroke; concomitant use of other medications that lower the seizure threshold (e.g., other bupropion products, antipsychotics, tricyclic antidepressants, theophylline, and systemic corticosteroids), metabolic disorders (e.g., hypoglycemia, hyponatremia, severe hepatic impairment, and hypoxia), use of illicit drugs (e.g., cocaine), or abuse or misuse of prescription drugs such as CNS stimulants. Additional predisposing conditions include diabetes mellitus treated with oral hypoglycemic drugs or insulin; use of anorectic drugs; and excessive use of alcohol, benzodiazepines, sedative/hypnotics, or opiates.
Incidence of Seizure With Bupropion Use: Doses for smoking cessation should not exceed 300 mg per day. The seizure rate associated with doses of sustained-release bupropion in depressed patients up to 300 mg per day is approximately 0.1% (1/1,000) and increases to approximately 0.4% (4/1000) at doses up to 400 mg per day.
The risk of seizure can be reduced if the dose of BUPROBAN® for smoking cessation does not exceed 300 mg per day, given as 150 mg twice daily, and titration rate is gradual.
5.4 Hypertension
Treatment with BUPROBAN® can result in elevated blood pressure and hypertension. Assess blood pressure before initiating treatment with BUPROBAN®, and monitor periodically during treatment. The risk of hypertension is increased if BUPROBAN® is used concomitantly with MAOIs or other drugs that increase dopaminergic or noradrenergic activity [see Contraindications (4)].
Data from a comparative trial of BUPROBAN®, nicotine transdermal system (NTS), the combination of BUPROBAN® plus NTS, and placebo as an aid to smoking cessation suggest a higher incidence of treatment-emergent hypertension in patients treated with the combination of BUPROBAN® and NTS. In this trial, 6.1% of subjects treated with the combination of BUPROBAN® and NTS had treatment-emergent hypertension compared to 2.5%, 1.6%, and 3.1% of subjects treated with BUPROBAN®, NTS, and placebo, respectively. The majority of these subjects had evidence of pre-existing hypertension. Three subjects (1.2%) treated with the combination of BUPROBAN® and NTS and 1 subject (0.4%) treated with NTS had study medication discontinued due to hypertension compared with none of the subjects treated with BUPROBAN® or placebo. Monitoring of blood pressure is recommended in patients who receive the combination of bupropion and nicotine replacement.
In a clinical trial of bupropion immediate-release in MDD subjects with stable congestive heart failure (N = 36), bupropion was associated with an exacerbation of pre-existing hypertension in 2 subjects, leading to discontinuation of bupropion treatment. There are no controlled trials assessing the safety of bupropion in patients with a recent history of myocardial infarction or unstable cardiac disease.
5.5 Activation of Mania/Hypomania
Antidepressant treatment can precipitate a manic, mixed, or hypomanic episode. The risk appears to be increased in patients with bipolar disorder or who have risk factors for bipolar disorder. There were no reports of activation of psychosis or mania in clinical trials with BUPROBAN® conducted in nondepressed smokers. Bupropion is not approved for use in treating bipolar depression.
5.6 Psychosis and Other Neuropsychiatric Reactions
Depressed patients treated with bupropion in depression trials have had a variety of neuropsychiatric signs and symptoms, including delusions, hallucinations, psychosis, concentration disturbance, paranoia, and confusion. Some of these patients had a diagnosis of bipolar disorder. In some cases, these symptoms abated upon dose reduction and/or withdrawal of treatment. Instruct patients to contact a healthcare professional if such reactions occur.
In clinical trials with BUPROBAN® conducted in nondepressed smokers, the incidence of neuropsychiatric side effects was generally comparable to placebo. However, in the postmarketing experience, patients taking BUPROBAN® to quit smoking have reported similar types of neuropsychiatric symptoms to those reported by patients in the clinical trials of bupropion for depression.
5.7 Hypersensitivity Reactions
Anaphylactoid/anaphylactic reactions have occurred during clinical trials with bupropion. Reactions have been characterized by pruritus, urticaria, angioedema, and dyspnea requiring medical treatment. In addition, there have been rare, spontaneous postmarketing reports of erythema multiforme, Stevens-Johnson syndrome, and anaphylactic shock associated with bupropion. Instruct patients to discontinue BUPROBAN® and consult a healthcare provider if they develop an allergic or anaphylactoid/anaphylactic reaction (e.g., skin rash, pruritus, hives, chest pain, edema, and shortness of breath) during treatment.
There are reports of arthralgia, myalgia, fever with rash and other serum sickness-like symptoms suggestive of delayed hypersensitivity.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Neuropsychiatric symptoms and suicide risk in smoking cessation treatment [see Boxed Warning and Warnings and Precautions (5.1)]
- Suicidal thoughts and behaviors in adolescents and young adults [see Boxed Warning and Warnings and Precautions (5.2)]
- Seizure [see Warnings and Precautions (5.3)]
- Hypertension [see Warnings and Precautions (5.4)]
- Activation of mania or hypomania [see Warnings and Precautions (5.5)]
- Psychosis and other neuropsychiatric reactions [see Warnings and Precautions (5.6)]
- Hypersensitivity reactions [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions Leading to Discontinuation of Treatment: Adverse reactions were sufficiently troublesome to cause discontinuation of treatment in 8% of the 706 subjects treated with BUPROBAN® and 5% of the 313 patients treated with placebo. The more common events leading to discontinuation of treatment with BUPROBAN® included nervous system disturbances (3.4%), primarily tremors, and skin disorders (2.4%), primarily rashes.
Commonly Observed Adverse Reactions: The most commonly observed adverse reactions consistently associated with the use of BUPROBAN® were dry mouth and insomnia. The incidence of dry mouth and insomnia may be related to the dose of BUPROBAN®. The occurrence of these adverse reactions may be minimized by reducing the dose of BUPROBAN®. In addition, insomnia may be minimized by avoiding bedtime doses.
Adverse reactions reported in the dose-response and comparator trials are presented in Table 2 and Table 3, respectively. Reported adverse reactions were classified using a COSTART-based dictionary.
| Adverse Reaction | BUPROBAN® 100 to 300 mg/day (n = 461) % |
Placebo (n = 150) % |
|---|---|---|
| Body (General) | ||
| Neck pain | 2 | <1 |
| Allergic reaction | 1 | 0 |
| Cardiovascular | ||
| Hot flashes | 1 | 0 |
| Hypertension | 1 | <1 |
| Digestive | ||
| Dry mouth | 11 | 5 |
| Increased appetite | 2 | <1 |
| Anorexia | 1 | <1 |
| Musculoskeletal | ||
| Arthralgia | 4 | 3 |
| Myalgia | 2 | 1 |
| Nervous system | ||
| Insomnia | 31 | 21 |
| Dizziness | 8 | 7 |
| Tremor | 2 | 1 |
| Somnolence | 2 | 1 |
| Thinking abnormality | 1 | 0 |
| Respiratory | ||
| Bronchitis | 2 | 0 |
| Skin | ||
| Pruritus | 3 | <1 |
| Rash | 3 | <1 |
| Dry skin | 2 | 0 |
| Urticaria | 1 | 0 |
| Special senses | ||
| Taste perversion | 2 | <1 |
| Adverse Experience (COSTART Term) |
BUPROBAN® 300 mg/day (n = 243) % |
Nicotine Transdermal System (NTS) 21 mg/day (n = 243) % |
BUPROBAN® and NTS (n = 244) % |
Placebo (n = 159) % |
|---|---|---|---|---|
| Body | ||||
| Abdominal pain | 3 | 4 | 1 | 1 |
| Accidental injury | 2 | 2 | 1 | 1 |
| Chest pain | <1 | 1 | 3 | 1 |
| Neck pain | 2 | 1 | <1 | 0 |
| Facial edema | <1 | 0 | 1 | 0 |
| Cardiovascular | ||||
| Hypertension | 1 | <1 | 2 | 0 |
| Palpitations | 2 | 0 | 1 | 0 |
| Digestive | ||||
| Nausea | 9 | 7 | 11 | 4 |
| Dry mouth | 10 | 4 | 9 | 4 |
| Constipation | 8 | 4 | 9 | 3 |
| Diarrhea | 4 | 4 | 3 | 1 |
| Anorexia | 3 | 1 | 5 | 1 |
| Mouth ulcer | 2 | 1 | 1 | 1 |
| Thirst | <1 | <1 | 2 | 0 |
| Musculoskeletal | ||||
| Myalgia | 4 | 3 | 5 | 3 |
| Arthralgia | 5 | 3 | 3 | 2 |
| Nervous system | ||||
| Insomnia | 40 | 28 | 45 | 18 |
| Dream abnormality | 5 | 18 | 13 | 3 |
| Anxiety | 8 | 6 | 9 | 6 |
| Disturbed concentration | 9 | 3 | 9 | 4 |
| Dizziness | 10 | 2 | 8 | 6 |
| Nervousness | 4 | <1 | 2 | 2 |
| Tremor | 1 | <1 | 2 | 0 |
| Dysphoria | <1 | 1 | 2 | 1 |
| Respiratory | ||||
| Rhinitis | 12 | 11 | 9 | 8 |
| Increased cough | 3 | 5 | <1 | 1 |
| Pharyngitis | 3 | 2 | 3 | 0 |
| Sinusitis | 2 | 2 | 2 | 1 |
| Dyspnea | 1 | 0 | 2 | 1 |
| Epistaxis | 2 | 1 | 1 | 0 |
| Skin | ||||
| Application site reaction |
11 | 17 | 15 | 7 |
| Rash | 4 | 3 | 3 | 2 |
| Pruritus | 3 | 1 | 5 | 1 |
| Urticaria | 2 | 0 | 2 | 0 |
| Special Senses | ||||
| Taste perversion | 3 | 1 | 3 | 2 |
| Tinnitus | 1 | 0 | <1 | 0 |
Adverse reactions in a 1-year maintenance trial and a 12-week COPD trial with BUPROBAN® were quantitatively and qualitatively similar to those observed in the dose-response and comparator trials.
Other Adverse Reactions Observed During the Clinical Development of Bupropion: In addition to the adverse reactions noted above, the following adverse reactions have been reported in clinical trials with the sustained-release formulation of bupropion in depressed subjects and in nondepressed smokers, as well as in clinical trials with the immediate-release formulation of bupropion.
Adverse reaction frequencies represent the proportion of subjects who experienced a treatment-emergent adverse reaction on at least one occasion in placebo-controlled trials for depression (n = 987) or smoking cessation (n = 1,013), or subjects who experienced an adverse reaction requiring discontinuation of treatment in an open-label surveillance trial with bupropion sustained-release tablets (n = 3,100). All treatment-emergent adverse reactions are included except those listed in Tables 2 and 3, those listed in other safety-related sections of the prescribing information, those subsumed under COSTART terms that are either overly general or excessively specific so as to be uninformative, those not reasonably associated with the use of the drug, and those that were not serious and occurred in fewer than 2 subjects.
Adverse reactions are further categorized by body system and listed in order of decreasing frequency according to the following definitions of frequency: Frequent adverse reactions are defined as those occurring in at least 1/100 subjects. Infrequent adverse reactions are those occurring in 1/100 to 1/1,000 subjects, while rare events are those occurring in less than 1/1,000 subjects.
Body (General): Frequent were asthenia, fever, and headache. Infrequent were chills, inguinal hernia, and photosensitivity. Rare was malaise.
Cardiovascular: Infrequent were flushing, migraine, postural hypotension, stroke, tachycardia, and vasodilation. Rare was syncope.
Digestive: Frequent were dyspepsia and vomiting. Infrequent were abnormal liver function, bruxism, dysphagia, gastric reflux, gingivitis, jaundice, and stomatitis.
Hemic and Lymphatic: Infrequent was ecchymosis.
Metabolic and Nutritional: Infrequent were edema and peripheral edema.
Musculoskeletal: Infrequent were leg cramps and twitching.
Nervous System: Frequent were agitation, depression, and irritability. Infrequent were abnormal coordination, CNS stimulation, confusion, decreased libido, decreased memory, depersonalization, emotional lability, hostility, hyperkinesia, hypertonia, hypesthesia, paresthesia, suicidal ideation, and vertigo. Rare were amnesia, ataxia, derealization, and hypomania.
Respiratory: Rare was bronchospasm.
Skin: Frequent was sweating.
Special Senses: Frequent was blurred vision or diplopia. Infrequent were accommodation abnormality and dry eye.
Urogenital: Frequent was urinary frequency. Infrequent were impotence, polyuria, and urinary urgency.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of BUPROBAN® and are not described elsewhere in the label. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a relationship to drug exposure.
Body (General): Arthralgia, myalgia, and fever with rash and other symptoms suggestive of delayed hypersensitivity. These symptoms may resemble serum sickness.
Cardiovascular: Cardiovascular disorder, complete AV block, extrasystoles, hypotension, myocardial infarction, phlebitis, and pulmonary embolism.
Digestive: Colitis, esophagitis, gastrointestinal hemorrhage, gum hemorrhage, hepatitis, increased salivation, intestinal perforation, liver damage, pancreatitis, stomach ulcer, and stool abnormality.
Endocrine: Hyperglycemia, hypoglycemia, and syndrome of inappropriate antidiuretic hormone.
Hemic and Lymphatic: Anemia, leukocytosis, leukopenia, lymphadenopathy, pancytopenia, and thrombocytopenia. Altered PT and/or INR, infrequently associated with hemorrhagic or thrombotic complications, were observed when bupropion was coadministered with warfarin.
Metabolic and Nutritional: Glycosuria.
Musculoskeletal: Arthritis and muscle rigidity/fever/rhabdomyolysis, and muscle weakness.
Nervous System: Abnormal electroencephalogram (EEG), aggression, akinesia, aphasia, coma, completed suicide, delirium, delusions, dysarthria, dyskinesia, dystonia, euphoria, extrapyramidal syndrome, hallucinations, hypokinesia, increased libido, manic reaction, neuralgia, neuropathy, paranoid ideation, restlessness, suicide attempt, and unmasking tardive dyskinesia.
Respiratory: Pneumonia.
Skin: Alopecia, angioedema, exfoliative dermatitis, hirsutism, and Stevens-Johnson syndrome.
Special Senses: Deafness, increased intraocular pressure, and mydriasis.
Urogenital: Abnormal ejaculation, cystitis, dyspareunia, dysuria, gynecomastia, menopause, painful erection, prostate disorder, salpingitis, urinary incontinence, urinary retention, urinary tract disorder, and vaginitis.
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect BUPROBAN
Bupropion is primarily metabolized to hydroxybupropion by CYP2B6. Therefore, the potential exists for drug interactions between BUPROBAN® and drugs that are inhibitors or inducers of CYP2B6.
Inhibitors of CYP2B6: Ticlopidine and Clopidogrel: Concomitant treatment with these drugs can increase bupropion exposure but decrease hydroxybupropion exposure. Based on clinical response, dosage adjustment of BUPROBAN® may be necessary when coadministered with CYP2B6 inhibitors (e.g., ticlopidine or clopidogrel) [see Clinical Pharmacology (12.3)].
Inducers of CYP2B6: Ritonavir, Lopinavir, and Efavirenz: Concomitant treatment with these drugs can decrease bupropion and hydroxybupropion exposure. Dosage increase of BUPROBAN® may be necessary when coadministered with ritonavir, lopinavir, or efavirenz [see Clinical Pharmacology (12.3)] but should not exceed the maximum recommended dose.
Carbamazepine, Phenobarbital, Phenytoin: While not systematically studied, these drugs may induce the metabolism of bupropion and may decrease bupropion exposure [see Clinical Pharmacology (12.3)]. If bupropion is used concomitantly with a CYP inducer, it may be necessary to increase the dose of bupropion, but the maximum recommended dose should not be exceeded.
7.2 Potential for BUPROBAN to Affect Other Drugs
Drugs Metabolized by CYP2D6: Bupropion and its metabolites (erythrohydrobupropion, threohydrobupropion, hydroxybupropion) are CYP2D6 inhibitors. Therefore, coadministration of BUPROBAN® with drugs that are metabolized by CYP2D6 can increase the exposures of drugs that are substrates of CYP2D6. Such drugs include certain antidepressants (e.g., venlafaxine, nortriptyline, imipramine, desipramine, paroxetine, fluoxetine, and sertraline), antipsychotics (e.g., haloperidol, risperidone, thioridazine), beta-blockers (e.g., metoprolol), and Type 1C antiarrhythmics (e.g., propafenone and flecainide). When used concomitantly with BUPROBAN®, it may be necessary to decrease the dose of these CYP2D6 substrates, particularly for drugs with a narrow therapeutic index.
Drugs that require metabolic activation by CYP2D6 to be effective (e.g., tamoxifen) theoretically could have reduced efficacy when administered concomitantly with inhibitors of CYP2D6 such as bupropion. Patients treated concomitantly with BUPROBAN® and such drugs may require increased doses of the drug [see Clinical Pharmacology (12.3)].
7.3 Drugs That Lower Seizure Threshold
Use extreme caution when coadministering BUPROBAN® with other drugs that lower seizure threshold (e.g., other bupropion products, antipsychotics, antidepressants, theophylline, or systemic corticosteroids). Use low initial doses and increase the dose gradually [see Contraindications (4) and Warnings and Precautions (5.3)].
7.4 Dopaminergic Drugs (Levodopa and Amantadine)
Bupropion, levodopa, and amantadine have dopamine agonist effects. CNS toxicity has been reported when bupropion was coadministered with levodopa or amantadine. Adverse reactions have included restlessness, agitation, tremor, ataxia, gait disturbance, vertigo, and dizziness. It is presumed that the toxicity results from cumulative dopamine agonist effects. Use caution when administering BUPROBAN® concomitantly with these drugs.
7.5 Use With Alcohol
In postmarketing experience, there have been rare reports of adverse neuropsychiatric events or reduced alcohol tolerance in patients who were drinking alcohol during treatment with BUPROBAN®. The consumption of alcohol during treatment with BUPROBAN® should be minimized or avoided.
7.6 MAO Inhibitors
Bupropion inhibits the reuptake of dopamine and norepinephrine. Concomitant use of MAOIs and bupropion is contraindicated because there is an increased risk of hypertensive reactions if bupropion is used concomitantly with MAOIs. Studies in animals demonstrate that the acute toxicity of bupropion is enhanced by the MAO inhibitor phenelzine. At least 14 days should elapse between discontinuation of an MAOI and initiation of treatment with BUPROBAN®. Conversely, at least 14 days should be allowed after stopping BUPROBAN® before starting an MAOI intended to treat psychiatric disorders [see Dosage and Administration (2.8) and Contraindications (4)].
7.7 Smoking Cessation
Physiological changes resulting from smoking cessation, with or without treatment with BUPROBAN®, may alter the pharmacokinetics or pharmacodynamics of certain drugs (e.g., theophylline, warfarin, insulin) for which dosage adjustment may be necessary.
7.8 Drug-Laboratory Test Interactions
False-positive urine immunoassay screening tests for amphetamines have been reported in patients taking bupropion. This is due to lack of specificity of some screening tests. False-positive test results may result even following discontinuation of bupropion therapy. Confirmatory tests, such as gas chromatography/mass spectrometry, will distinguish bupropion from amphetamines.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
Risk Summary: Data from epidemiological studies of pregnant women exposed to bupropion in the first trimester indicate no increased risk of congenital malformations overall. All pregnancies, regardless of drug exposure, have a background rate of 2% to 4% for major malformations, and 15% to 20% for pregnancy loss. No clear evidence of teratogenic activity was found in reproductive developmental studies conducted in rats and rabbits; however, in rabbits, slightly increased incidences of fetal malformations and skeletal variations were observed at doses approximately 2 times the maximum recommended human dose (MRHD) and greater and decreased fetal weights were seen at doses three times the MRHD and greater. BUPROBAN® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations: Pregnant smokers should be encouraged to attempt cessation using educational and behavioral interventions before pharmacological approaches are used.
Human Data: Data from the international bupropion Pregnancy Registry (675 first trimester exposures) and a retrospective cohort study using the United Healthcare database (1,213 first trimester exposures) did not show an increased risk for malformations overall.
No increased risk for cardiovascular malformations overall has been observed after bupropion exposure during the first trimester. The prospectively observed rate of cardiovascular malformations in pregnancies with exposure to bupropion in the first trimester from the international Pregnancy Registry was 1.3% (9 cardiovascular malformations/675 first trimester maternal bupropion exposures), which is similar to the background rate of cardiovascular malformations (approximately 1%). Data from the United Healthcare database and a case-control study (6,853 infants with cardiovascular malformations and 5,763 with non-cardiovascular malformations) from the National Birth Defects Prevention Study (NBDPS) did not show an increased risk for cardiovascular malformations overall after bupropion exposure during the first trimester.
Study findings on bupropion exposure during the first trimester and risk for left ventricular outflow tract obstruction (LVOTO) are inconsistent and do not allow conclusions regarding a possible association. The United Healthcare database lacked sufficient power to evaluate this association; the NBDPS found increased risk for LVOTO (n = 10; adjusted OR = 2.6; 95% CI: 1.2, 5.7), and the Slone Epidemiology case control study did not find increased risk for LVOTO.
Study findings on bupropion exposure during the first trimester and risk for ventricular septal defect (VSD) are inconsistent and do not allow conclusions regarding a possible association. The Slone Epidemiology Study found an increased risk for VSD following first trimester maternal bupropion exposure (n = 17; adjusted OR = 2.5; 95% CI: 1.3, 5.0) but did not find increased risk for any other cardiovascular malformations studied (including LVOTO as above). The NBDPS and United Healthcare database study did not find an association between first trimester maternal bupropion exposure and VSD.
For the findings of LVOTO and VSD, the studies were limited by the small number of exposed cases, inconsistent findings among studies, and the potential for chance findings from multiple comparisons in case control studies.
Animal Data: In studies conducted in rats and rabbits, bupropion was administered orally during the period of organogenesis at doses of up to 450 and 150 mg/kg/day, respectively (approximately 15 and 10 times the MRHD respectively, on a mg/m2 basis). No clear evidence of teratogenic activity was found in either species; however, in rabbits, slightly increased incidences of fetal malformations and skeletal variations were observed at the lowest dose tested (25 mg/kg/day, approximately 2 times the MRHD on a mg/m2 basis) and greater. Decreased fetal weights were observed at 50 mg/kg and greater.
When rats were administered bupropion at oral doses of up to 300 mg/kg/day (approximately 10 times the MRHD on a mg/m2 basis) prior to mating and throughout pregnancy and lactation, there were no apparent adverse effects on offspring development.
8.3 Nursing Mothers
Bupropion and its metabolites are present in human milk. In a lactation study of 10 women, levels of orally dosed bupropion and its active metabolites were measured in expressed milk. The average daily infant exposure (assuming 150 mL/kg daily consumption) to bupropion and its active metabolites was 2% of the maternal weight-adjusted dose. Exercise caution when BUPROBAN® is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in the pediatric population have not been established [see Boxed Warning and Warnings and Precautions (5.2)].
8.5 Geriatric Use
Of the approximately 6,000 subjects who participated in clinical trials with bupropion sustained-release tablets (depression and smoking cessation trials), 275 were aged ≥65 years and 47 were aged ≥75 years. In addition, several hundred subjects aged ≥65 years participated in clinical trials using the immediate-release formulation of bupropion (depression trials). No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Bupropion is extensively metabolized in the liver to active metabolites, which are further metabolized and excreted by the kidneys. The risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be necessary to consider this factor in dose selection; it may be useful to monitor renal function [see Dosage and Administration (2.7), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Consider a reduced dose and/or dosing frequency of BUPROBAN® in patients with renal impairment (Glomerular Filtration Rate: <90 mL/min). Bupropion and its metabolites are cleared renally and may accumulate in such patients to a greater extent than usual. Monitor closely for adverse reactions that could indicate high bupropion or metabolite exposures [see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
In patients with moderate to severe hepatic impairment (Child-Pugh score: 7 to 15), the maximum dose of BUPROBAN® is 150 mg every other day. In patients with mild hepatic impairment (Child-Pugh score: 5 to 6), consider reducing the dose and/or frequency of dosing [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Bupropion is not a controlled substance.
9.2 Abuse
Humans: Controlled clinical trials of bupropion (immediate-release formulation) conducted in normal volunteers, in subjects with a history of multiple drug abuse, and in depressed subjects showed some increase in motor activity and agitation/excitement.
In a population of individuals experienced with drugs of abuse, a single dose of 400 mg of bupropion produced mild amphetamine-like activity as compared with placebo on the Morphine-Benzedrine Subscale of the Addiction Research Center Inventories (ARCI) and a score intermediate between placebo and amphetamine on the Liking Scale of the ARCI. These scales measure general feelings of euphoria and drug desirability.
Findings in clinical trials, however, are not known to reliably predict the abuse potential of drugs. Nonetheless, evidence from single-dose trials does suggest that the recommended daily dosage of bupropion when administered in divided doses is not likely to be significantly reinforcing to amphetamine or CNS stimulant abusers. However, higher doses (that could not be tested because of the risk of seizure) might be modestly attractive to those who abuse CNS stimulant drugs.
Animals: Studies in rodents and primates demonstrated that bupropion exhibits some pharmacologic actions common to psychostimulants. In rodents, it has been shown to increase locomotor activity, elicit a mild stereotyped behavior response, and increase rates of responding in several schedule-controlled behavior paradigms. In primate models assessing the positive reinforcing effects of psychoactive drugs, bupropion was self-administered intravenously. In rats, bupropion produced amphetamine-like and cocaine-like discriminative stimulus effects in drug discrimination paradigms used to characterize the subjective effects of psychoactive drugs.
The possibility that bupropion may induce dependence should be kept in mind when evaluating the desirability of including the drug in smoking cessation programs of individual patients.
10 OVERDOSAGE
10.1 Human Overdose Experience
Overdoses of up to 30 grams or more of bupropion have been reported. Seizure was reported in approximately one-third of all cases. Other serious reactions reported with overdoses of bupropion alone included hallucinations, loss of consciousness, sinus tachycardia, and ECG changes such as conduction disturbances (including QRS prolongation) or arrhythmias. Fever, muscle rigidity, rhabdomyolysis, hypotension, stupor, coma, and respiratory failure have been reported mainly when bupropion was part of multiple drug overdoses.
Although most patients recovered without sequelae, deaths associated with overdoses of bupropion alone have been reported in patients ingesting large doses of the drug. Multiple uncontrolled seizures, bradycardia, cardiac failure, and cardiac arrest prior to death were reported in these patients.
10.2 Overdosage Management
Consult a Certified Poison Control Center for up-to-date guidance and advice. Telephone numbers for certified poison control centers are listed in the Physicians' Desk Reference (PDR). Call 1-800-222-1222 or refer to www.poison.org.
There are no known antidotes for bupropion. In case of an overdose, provide supportive care, including close medical supervision and monitoring. Consider the possibility of multiple drug overdose. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. Induction of emesis is not recommended.
11 DESCRIPTION
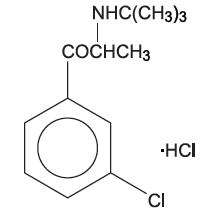
BUPROBAN® is a non-nicotine aid to smoking cessation. BUPROBAN® is chemically unrelated to nicotine or other agents currently used in the treatment of nicotine addiction. Initially developed and marketed as an antidepressant (WELLBUTRIN® [bupropion hydrochloride] Tablets and WELLBUTRIN SR® [bupropion hydrochloride] Sustained-Release Tablets), BUPROBAN® is also chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that of diethylpropion; it is related to phenylethylamines. It is designated as (±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propanone hydrochloride. The molecular weight is 276.2. The molecular formula is C13H18ClNO∙HCl. Bupropion hydrochloride powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral mucosa. The structural formula is:
BUPROBAN® is supplied for oral administration as 150-mg, film-coated, extended-release tablets. Each tablet contains the labeled amount of bupropion hydrochloride and the inactive ingredients: colloidal silicone dioxide, hydroxypropylcellulose, hypromellose, iron oxide yellow, macrogol, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide and triacetin.
This product meets USP Drug Release Test #3.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism by which BUPROBAN® enhances the ability of patients to abstain from smoking is not known but is presumed to be related to noradrenergic and/or dopaminergic mechanisms. Bupropion is a relatively weak inhibitor of the neuronal reuptake of norepinephrine and dopamine, and does not inhibit the reuptake of serotonin. Bupropion does not inhibit monoamine oxidase.
12.3 Pharmacokinetics
Bupropion is a racemic mixture. The pharmacological activity and pharmacokinetics of the individual enantiomers have not been studied. The mean elimination half-life (±SD) of bupropion after chronic dosing is 21 (±9) hours, and steady-state plasma concentrations of bupropion are reached within 8 days.
Absorption: The absolute bioavailability of BUPROBAN® in humans has not been determined because an intravenous formulation for human use is not available. However, it appears likely that only a small proportion of any orally administered dose reaches the systemic circulation intact. In rat and dog studies, the bioavailability of bupropion ranged from 5% to 20%.
In humans, following oral administration of BUPROBAN®, peak plasma concentration (Cmax) of bupropion is usually achieved within 3 hours.
BUPROBAN® can be taken with or without food. Bupropion Cmax and AUC was increased by 11% to 35%, and 16% to 19%, respectively, when BUPROBAN® was administered with food to healthy volunteers in three trials. The food effect is not considered clinically significant.
Distribution: In vitro tests show that bupropion is 84% bound to human plasma proteins at concentrations up to 200 mcg/mL. The extent of protein binding of the hydroxybupropion metabolite is similar to that for bupropion; whereas, the extent of protein binding of the threohydrobupropion metabolite is about half that seen with bupropion.
Metabolism: Bupropion is extensively metabolized in humans. Three metabolites are active: hydroxybupropion, which is formed via hydroxylation of the tert-butyl group of bupropion, and the amino-alcohol isomers, threohydrobupropion and erythrohydrobupropion, which are formed via reduction of the carbonyl group. In vitro findings suggest that CYP2B6 is the principal isoenzyme involved in the formation of hydroxybupropion, while cytochrome P450 enzymes are not involved in the formation of threohydrobupropion. Oxidation of the bupropion side chain results in the formation of a glycine conjugate of meta-chlorobenzoic acid, which is then excreted as the major urinary metabolite. The potency and toxicity of the metabolites relative to bupropion have not been fully characterized. However, it has been demonstrated in an antidepressant screening test in mice that hydroxybupropion is one-half as potent as bupropion, while threohydrobupropion and erythrohydrobupropion are 5-fold less potent than bupropion. This may be of clinical importance because the plasma concentrations of the metabolites are as high as or higher than those of bupropion.
Following a single dose administration of BUPROBAN® in humans, Cmax of hydroxybupropion occurs approximately 6 hours post-dose and is approximately 10 times the peak level of the parent drug at steady state. The elimination half-life of hydroxybupropion is approximately 20 (±5) hours and its AUC at steady state is about 17 times that of bupropion. The times to peak concentrations for the erythrohydrobupropion and threohydrobupropion metabolites are similar to that of the hydroxybupropion metabolite. However, their elimination half-lives are longer, 33 (±10) and 37 (±13) hours, respectively, and steady-state AUCs are 1.5 and 7 times that of bupropion, respectively.
Bupropion and its metabolites exhibit linear kinetics following chronic administration of 300 to 450 mg per day.
Elimination: Following oral administration of 200 mg of 14C-bupropion in humans, 87% and 10% of the radioactive dose were recovered in the urine and feces, respectively. Only 0.5% of the oral dose was excreted as unchanged bupropion.
Population Subgroups: Factors or conditions altering metabolic capacity (e.g., liver disease, congestive heart failure [CHF], age, concomitant medications, etc.) or elimination may be expected to influence the degree and extent of accumulation of the active metabolites of bupropion. The elimination of the major metabolites of bupropion may be affected by reduced renal or hepatic function because they are moderately polar compounds and are likely to undergo further metabolism or conjugation in the liver prior to urinary excretion.
Renal Impairment: There is limited information on the pharmacokinetics of bupropion in patients with renal impairment. An inter-trial comparison between normal subjects and subjects with end-stage renal failure demonstrated that the parent drug Cmax and AUC values were comparable in the 2 groups, whereas the hydroxybupropion and threohydrobupropion metabolites had a 2.3- and 2.8-fold increase, respectively, in AUC for subjects with end-stage renal failure. A second trial, comparing normal subjects and subjects with moderate-to-severe renal impairment (GFR 30.9 ± 10.8 mL/min), showed that after a single 150-mg dose of sustained-release bupropion, exposure to bupropion was approximately 2-fold higher in subjects with impaired renal function while levels of the hydroxybupropion and threo/erythrohydrobupropion (combined) metabolites were similar in the 2 groups. Bupropion is extensively metabolized in the liver to active metabolites, which are further metabolized and subsequently excreted by the kidneys. The elimination of the major metabolites of bupropion may be reduced by impaired renal function. BUPROBAN® should be used with caution in patients with renal impairment and a reduced frequency and/or dose should be considered [see Use in Specific Populations (8.6)].
Hepatic Impairment: The effect of hepatic impairment on the pharmacokinetics of bupropion was characterized in 2 single-dose trials, one in subjects with alcoholic liver disease and one in subjects with mild-to-severe cirrhosis. The first trial demonstrated that the half-life of hydroxybupropion was significantly longer in 8 subjects with alcoholic liver disease than in 8 healthy volunteers (32 ± 14 hours versus 21 ± 5 hours, respectively). Although not statistically significant, the AUCs for bupropion and hydroxybupropion were more variable and tended to be greater (by 53% to 57%) in volunteers with alcoholic liver disease. The differences in half-life for bupropion and the other metabolites in the 2 groups were minimal.
The second trial demonstrated no statistically significant differences in the pharmacokinetics of bupropion and its active metabolites in 9 subjects with mild-to-moderate hepatic cirrhosis compared with 8 healthy volunteers. However, more variability was observed in some of the pharmacokinetic parameters for bupropion (AUC, Cmax, and Tmax) and its active metabolites (t½) in subjects with mild-to-moderate hepatic cirrhosis. In 8 subjects with severe hepatic cirrhosis, significant alterations in the pharmacokinetics of bupropion and its metabolites were seen (Table 4).
| Cmax | AUC | t½ | Tmax
|
|
|---|---|---|---|---|
| Bupropion | 1.69 | 3.12 | 1.43 | 0.5 h |
| Hydroxybupropion | 0.31 | 1.28 | 3.88 | 19 h |
| Threo/erythrohydrobupropion amino alcohol | 0.69 | 2.48 | 1.96 | 20 h |
Smokers: The effects of cigarette smoking on the pharmacokinetics of bupropion were studied in 34 healthy male and female volunteers; 17 were chronic cigarette smokers and 17 were nonsmokers. Following oral administration of a single 150-mg dose of BUPROBAN®, there were no statistically significant differences in Cmax, half-life, Tmax, AUC, or clearance of bupropion or its major metabolites between smokers and nonsmokers.
In a trial comparing the treatment combination of BUPROBAN® and NTS versus BUPROBAN® alone, no statistically significant differences were observed between the 2 treatment groups of combination BUPROBAN® and NTS (n = 197) and BUPROBAN® alone (n = 193) in the plasma concentrations of bupropion or its active metabolites at Weeks 3 and 6.
Left Ventricular Dysfunction: During a chronic dosing trial with bupropion in 14 depressed subjects with left ventricular dysfunction (history of CHF or an enlarged heart on x-ray), there was no apparent effect on the pharmacokinetics of bupropion or its metabolites, compared with healthy volunteers.
Age: The effects of age on the pharmacokinetics of bupropion and its metabolites have not been fully characterized, but an exploration of steady-state bupropion concentrations from several depression efficacy trials involving subjects dosed in a range of 300 to 750 mg per day, on a 3-times-daily schedule, revealed no relationship between age (18 to 83 years) and plasma concentration of bupropion. A single-dose pharmacokinetic trial demonstrated that the disposition of bupropion and its metabolites in elderly subjects was similar to that of younger subjects. These data suggest there is no prominent effect of age on bupropion concentration; however, another single- and multiple-dose pharmacokinetics trial suggested that the elderly are at increased risk for accumulation of bupropion and its metabolites [see Use in Specific Populations (8.5)].
Gender: Pooled analysis of bupropion pharmacokinetic data from 90 healthy male and 90 healthy female volunteers revealed no sex-related differences in the peak plasma concentrations of bupropion. The mean systemic exposure (AUC) was approximately 13% higher in male volunteers compared with female volunteers. The clinical significance of this finding is unknown.
Drug Interactions: Potential for Other Drugs to Affect BUPROBAN ® : In vitro studies indicate that bupropion is primarily metabolized to hydroxybupropion by CYP2B6. Therefore, the potential exists for drug interactions between BUPROBAN® and drugs that are inhibitors or inducers of CYP2B6. In addition, in vitro studies suggest that paroxetine, sertraline, norfluoxetine, fluvoxamine, and nelfinavir inhibit the hydroxylation of bupropion.
Inhibitors of CYP2B6: Ticlopidine, Clopidogrel: In a trial in healthy male volunteers, clopidogrel 75 mg once daily or ticlopidine 250 mg twice daily increased exposures (Cmax and AUC) of bupropion by 40% and 60% for clopidogrel, and by 38% and 85% for ticlopidine, respectively. The exposures (Cmax and AUC) of hydroxybupropion were decreased 50% and 52%, respectively, by clopidogrel, and 78% and 84%, respectively, by ticlopidine. This effect is thought to be due to the inhibition of the CYP2B6-catalyzed bupropion hydroxylation.
Prasugrel: Prasugrel is a weak inhibitor of CYP2B6. In healthy subjects, prasugrel increased bupropion Cmax and AUC values by 14% and 18%, respectively, and decreased Cmax and AUC values of hydroxybupropion, an active metabolite of bupropion, by 32% and 24%, respectively.
Cimetidine: The threohydrobupropion metabolite of bupropion does not appear to be produced by cytochrome P450 enzymes. The effects of concomitant administration of cimetidine on the pharmacokinetics of bupropion and its active metabolites were studied in 24 healthy young male volunteers. Following oral administration of bupropion 300 mg with and without cimetidine 800 mg, the pharmacokinetics of bupropion and hydroxybupropion were unaffected. However, there were 16% and 32% increases in the AUC and Cmax, respectively, of the combined moieties of threohydrobupropion and erythrohydrobupropion.
Citalopram: Citalopram did not affect the pharmacokinetics of bupropion and its 3 metabolites.
Inducers of CYP2B6: Ritonavir and Lopinavir: In a healthy volunteer trial, ritonavir 100 mg twice daily reduced the AUC and Cmax of bupropion by 22% and 21%, respectively. The exposure of the hydroxybupropion metabolite was decreased by 23%, the threohydrobupropion decreased by 38%, and the erythrohydrobupropion decreased by 48%.
In a second healthy volunteer trial, ritonavir at a dose of 600 mg twice daily decreased the AUC and the Cmax of bupropion by 66% and 62%, respectively. The exposure of the hydroxybupropion metabolite was decreased by 78%, the threohydrobupropion decreased by 50%, and the erythrohydrobupropion decreased by 68%.
In another healthy volunteer trial, lopinavir 400 mg/ritonavir 100 mg twice daily decreased bupropion AUC and Cmax by 57%. The AUC and Cmax of hydroxybupropion were decreased by 50% and 31%, respectively.
Efavirenz: In a trial in healthy volunteers, efavirenz 600 mg once daily for 2 weeks reduced the AUC and Cmax of bupropion by approximately 55% and 34%, respectively. The AUC of hydroxybupropion was unchanged, whereas Cmax of hydroxybupropion was increased by 50%.
Carbamazepine, Phenobarbital, Phenytoin: While not systematically studied, these drugs may induce the metabolism of bupropion.
Potential for BUPROBAN® to Affect Other Drugs: Animal data indicated that bupropion may be an inducer of drug-metabolizing enzymes in humans. In one trial, following chronic administration of bupropion 100 mg three times daily to 8 healthy male volunteers for 14 days, there was no evidence of induction of its own metabolism. Nevertheless, there may be potential for clinically important alterations of blood levels of co-administered drugs.
Drugs Metabolized by CYP2D6: In vitro, bupropion and its metabolites (erythrohydrobupropion, threohydrobupropion, hydroxybupropion) are CYP2D6 inhibitors. In a clinical trial of 15 male subjects (ages 19 to 35 years) who were extensive metabolizers of CYP2D6, bupropion 300 mg per day followed by a single dose of 50 mg desipramine increased the Cmax, AUC, and t1/2 of desipramine by an average of approximately 2-, 5- and 2-fold, respectively. The effect was present for at least 7 days after the last dose of bupropion. Concomitant use of bupropion with other drugs metabolized by CYP2D6 has not been formally studied.
Citalopram: Although citalopram is not primarily metabolized by CYP2D6, in one trial bupropion increased the Cmax and AUC of citalopram by 30% and 40%, respectively.
Lamotrigine: Multiple oral doses of bupropion had no statistically significant effects on the single-dose pharmacokinetics of lamotrigine in 12 healthy volunteers.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime carcinogenicity studies were performed in rats and mice at bupropion doses up to 300 and 150 mg/kg/day, respectively. These doses are approximately 10 and 2 times the MRHD, respectively, on a mg/m2 basis. In the rat study there was an increase in nodular proliferative lesions of the liver at doses of 100 to 300 mg/kg/day (approximately 3 to 10 times the MRHD on a mg/m2 basis); lower doses were not tested. The question of whether or not such lesions may be precursors of neoplasms of the liver is currently unresolved. Similar liver lesions were not seen in the mouse study, and no increase in malignant tumors of the liver and other organs was seen in either study.
Bupropion produced a positive response (2 to 3 times control mutation rate) in 2 of 5 strains in the Ames bacterial mutagenicity assay. Bupropion produced an increase in chromosomal aberrations in 1 of 3 in vivo rat bone marrow cytogenetic studies.
A fertility study in rats at doses up to 300 mg/kg/day revealed no evidence of impaired fertility.
14 CLINICAL STUDIES
The efficacy of BUPROBAN® as an aid to smoking cessation was demonstrated in 3 placebo-controlled, double-blind trials in nondepressed chronic cigarette smokers (n = 1,940, ≥15 cigarettes per day). In these trials, BUPROBAN® was used in conjunction with individual smoking cessation counseling.
The first trial was a dose-response trial conducted at 3 clinical centers. Subjects in this trial were treated for 7 weeks with 1 of 3 doses of BUPROBAN® (100, 150, or 300 mg per day) or placebo; quitting was defined as total abstinence during the last 4 weeks of treatment (Weeks 4 through 7). Abstinence was determined by subject daily diaries and verified by carbon monoxide levels in expired air.
Results of this dose-response trial with BUPROBAN® demonstrated a dose-dependent increase in the percentage of subjects able to achieve 4-week abstinence (Weeks 4 through 7). Treatment with BUPROBAN® at both 150 and 300 mg per day was significantly more effective than placebo in this trial.
Table 5 presents quit rates over time in the multicenter trial by treatment group. The quit rates are the proportions of all subjects initially enrolled (i.e., intent-to-treat analysis) who abstained from Week 4 of the trial through the specified week. Treatment with BUPROBAN® (150 or 300 mg per day) was more effective than placebo in helping subjects achieve 4-week abstinence. In addition, treatment with BUPROBAN® (7 weeks at 300 mg per day) was more effective than placebo in helping subjects maintain continuous abstinence through Week 26 (6 months) of the trial.
| Abstinence From Week 4 Through Specified Week | Treatment Groups | |||
|---|---|---|---|---|
| Placebo (n = 151) % (95% CI) |
BUPROBAN® 100 mg/day (n = 153) % (95% CI) |
BUPROBAN® 150 mg/day (n = 153) % (95% CI) |
BUPROBAN® 300 mg/day (n = 156) % (95% CI) |
|
| Week 7 (4-week quit) |
17% (11 to 23) |
22% (15 to 28) |
27% (20 to 35) |
36% (28 to 43) |
| Week 12 | 14% (8 to 19) |
20% (13 to 26) |
20% (14 to 27) |
25% (18 to 32) |
| Week 26 | 11% (6 to 16) |
16% (11 to 22) |
18% (12 to 24) |
19% (13 to 25) |
The second trial was a comparator trial conducted at 4 clinical centers. Four treatments were evaluated: BUPROBAN® 300 mg per day, nicotine transdermal system (NTS) 21 mg per day, combination of BUPROBAN® 300 mg per day plus NTS 21 mg per day, and placebo. Subjects were treated for 9 weeks. Treatment with BUPROBAN® was initiated at 150 mg per day while the subject was still smoking and was increased after 3 days to 300 mg per day given as 150 mg twice daily. NTS 21 mg per day was added to treatment with BUPROBAN® after approximately 1 week when the subject reached the target quit date. During Weeks 8 and 9 of the trial, NTS was tapered to 14 and 7 mg per day, respectively. Quitting, defined as total abstinence during Weeks 4 through 7, was determined by subject daily diaries and verified by expired air carbon monoxide levels. In this trial, subjects treated with any of the 3 treatments achieved greater 4-week abstinence rates than subjects treated with placebo.
Table 6 presents quit rates over time by treatment group for the comparator trial.
| Abstinence From Week 4 Through Specified Week | Treatment Groups | |||
|---|---|---|---|---|
| Placebo (n = 160) % (95% CI) |
Nicotine Transdermal System (NTS) 21 mg/day (n = 244) % (95% CI) |
BUPROBAN® 300 mg/day (n = 244) % (95% CI) |
BUPROBAN® 300 mg/day and NTS 21 mg/day (n = 245) % (95% CI) |
|
| Week 7 (4-week quit) |
23% (17 to 30) |
36% (30 to 42) |
49% (43 to 56) |
58% (51 to 64) |
| Week 10 | 20% (14 to 26) |
32% (26 to 37) |
46% (39 to 52) |
51% (45 to 58) |
When subjects in this trial were followed out to 1 year, the superiority of BUPROBAN® and the combination of BUPROBAN® and NTS over placebo in helping them to achieve abstinence from smoking was maintained. The continuous abstinence rate was 30% (95% CI: 24 to 35) in the subjects treated with BUPROBAN® and 33% (95% CI: 27 to 39) for subjects treated with the combination at 26 weeks compared with 13% (95% CI: 7 to 18) in the placebo group. At 52 weeks, the continuous abstinence rate was 23% (95% CI: 18 to 28) in the subjects treated with BUPROBAN® and 28% (95% CI: 23 to 34) for subjects treated with the combination, compared with 8% (95% CI: 3 to 12) in the placebo group. Although the treatment combination of BUPROBAN® and NTS displayed the highest rates of continuous abstinence throughout the trial, the quit rates for the combination were not significantly higher (P>0.05) than for BUPROBAN® alone.
The comparisons between BUPROBAN®, NTS, and combination treatment in this trial have not been replicated, and, therefore should not be interpreted as demonstrating the superiority of any of the active treatment arms over any other.
The third trial was a long-term maintenance trial conducted at 5 clinical centers. Subjects in this trial received open-label BUPROBAN® 300 mg per day for 7 weeks. Subjects who quit smoking while receiving BUPROBAN® (n = 432) were then randomized to BUPROBAN® 300 mg per day or placebo for a total trial duration of 1 year. Abstinence from smoking was determined by subject self-report and verified by expired air carbon monoxide levels. This trial demonstrated that at 6 months, continuous abstinence rates were significantly higher for subjects continuing to receive BUPROBAN® than for those switched to placebo (P<0.05; 55% versus 44%).
Quit rates in clinical trials are influenced by the population selected. Quit rates in an unselected population may be lower than the above rates. Quit rates for BUPROBAN® were similar in subjects with and without prior quit attempts using nicotine replacement therapy.
Treatment with BUPROBAN® reduced withdrawal symptoms compared with placebo. Reductions on the following withdrawal symptoms were most pronounced: irritability, frustration, or anger; anxiety; difficulty concentrating; restlessness; and depressed mood or negative affect. Depending on the trial and the measure used, treatment with BUPROBAN® showed evidence of reduction in craving for cigarettes or urge to smoke compared with placebo.
Use In Patients With Chronic Obstructive Pulmonary Disease (COPD): BUPROBAN® was evaluated in a randomized, double-blind, comparator trial of 404 subjects with mild-to-moderate COPD defined as FEV1≥35%, FEV1/FVC≤70%, and a diagnosis of chronic bronchitis, emphysema, and/or small airways disease. Subjects aged 36 to 76 years were randomized to BUPROBAN® 300 mg per day (n = 204) or placebo (n = 200) and treated for 12 weeks. Treatment with BUPROBAN® was initiated at 150 mg per day for 3 days while the subject was still smoking and increased to 150 mg twice daily for the remaining treatment period. Abstinence from smoking was determined by subject daily diaries and verified by carbon monoxide levels in expired air. Quitters were defined as subjects who were abstinent during the last 4 weeks of treatment. Table 7 shows quit rates in the COPD Trial.
| 4-Week Abstinence Period | Treatment Groups | |
|---|---|---|
| Placebo (n = 200) % (95% CI) |
BUPROBAN® 300 mg/day (n = 204) % (95% CI) |
|
| Weeks 9 through 12 | 12% (8 to 16) |
22% (17 to 27) |
16 HOW SUPPLIED/STORAGE AND HANDLING
BUPROBAN®, 150 mg of bupropion hydrochloride, is a light yellow, round, convex, film-coated, extended-release tablet debossed with "G" on one side and "2444" on the other side.
| Bottles of 100 | NDC 0093-5703-01 |
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in tightly-closed, light-resistant container (USP).
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Although BUPROBAN® is not indicated for treatment of depression, it contains the same active ingredient as the antidepressant medications WELLBUTRIN®, WELLBUTRIN SR®, and WELLBUTRIN XL®. Inform patients, their families, and their caregivers about the benefits and risks associated with treatment with BUPROBAN® and counsel them in its appropriate use.
A patient Medication Guide about "Quitting Smoking, Quit-Smoking Medications, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions," "Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions," and "What Other Important Information Should I Know About BUPROBAN®?" is available for BUPROBAN®. Instruct patients, their families, and their caregivers to read the Medication Guide and assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Advise patients regarding the following issues and to alert their prescriber if these occur while taking BUPROBAN®.
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment: Inform patients that quitting smoking, with or without BUPROBAN®, may be associated with nicotine withdrawal symptoms (including depression or agitation), or exacerbation of pre-existing psychiatric illness. Furthermore, some patients have experienced changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide when attempting to quit smoking while taking BUPROBAN®. If patients develop agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for them, or if patients develop suicidal ideation or behavior, they should be urged to report these symptoms to their healthcare provider immediately.
Suicidal Thoughts and Behaviors: Instruct patients, their families, and/or their caregivers to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Advise families and caregivers of patients to observe for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or healthcare professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Severe Allergic Reactions: Educate patients on the symptoms of hypersensitivity and to discontinue BUPROBAN® if they have a severe allergic reaction to BUPROBAN®.
Seizure: Instruct patients to discontinue BUPROBAN® and not restart it if they experience a seizure while on treatment. Advise patients that the excessive use or abrupt discontinuation of alcohol, benzodiazepines, antiepileptic drugs, or sedatives/hypnotics can increase the risk of seizure. Advise patients to minimize or avoid use of alcohol.
Bupropion-Containing Products: Educate patients that BUPROBAN® contains the same active ingredient (bupropion hydrochloride) found in WELLBUTRIN®, WELLBUTRIN SR®, and WELLBUTRIN XL®, which are used to treat depression and that BUPROBAN® should not be used in conjunction with any other medications that contain bupropion (such as WELLBUTRIN®, the immediate-release formulation; WELLBUTRIN SR®, the sustained-release formulation; WELLBUTRIN XL® or FORFIVO XL™, the extended-release formulations; and APLENZIN®, the extended-release formulation of bupropion hydrobromide). In addition, there are a number of generic bupropion HCl products for the immediate-, sustained-, and extended-release formulations.
Potential for Cognitive and Motor Impairment: Advise patients that any CNS-active drug like BUPROBAN® may impair their ability to perform tasks requiring judgment or motor and cognitive skills. Advise patients that until they are reasonably certain that BUPROBAN® does not adversely affect their performance, they should refrain from driving an automobile or operating complex, hazardous machinery. BUPROBAN® may lead to decreased alcohol tolerance.
Concomitant Medications: Counsel patients to notify their healthcare provider if they are taking or plan to take any prescription or over-the-counter drugs because BUPROBAN® and other drugs may affect each other's metabolisms.
Pregnancy: Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy.
Precautions for Nursing Mothers: Advise patients that BUPROBAN® is present in human milk in small amounts.
Storage Information: Instruct patients to store BUPROBAN® at room temperature, 20°C to 25°C (68°F to 77°F), and keep the tablets dry and out of the light.
Administration Information: Instruct patients to swallow BUPROBAN® Tablets whole so that the release rate is not altered. Do not chew, divide, or crush tablets; they are designed to slowly release drug in the body. When patients take more than 150 mg per day, instruct them to take BUPROBAN® in 2 doses at least 8 hours apart, to minimize the risk of seizures. Instruct patients if they miss a dose, not to take an extra tablet to make up for the missed dose and to take the next tablet at the regular time because of the dose-related risk of seizure. BUPROBAN® can be taken with or without food. Advise patients that BUPROBAN® Tablets may have an odor.
WELLBUTRIN®, WELLBUTRIN SR®, WELLBUTRIN XL® are registered trademarks of the GlaxoSmithKline group of companies.
FORFIVO XL™ is a registered trademark of Edgemont Pharmaceuticals, LLC.
APLENZIN® is a registered trademark of sanofi-aventis U.S. LLC.
Manufactured by:
Impax Laboratories, Inc.
Hayward, CA 94544 USA
Manufactured for:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
530-08
Rev. 04/2014
PHARMACIST - DETACH HERE AND GIVE MEDICATION GUIDE TO PATIENT.
MEDICATION GUIDE
BUPROBAN®
[buPROPion HCl Extended-Release Tablets (SR)]
Read this Medication Guide carefully before you start taking BUPROBAN® and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions about BUPROBAN®, ask your healthcare provider or pharmacist.
IMPORTANT: Be sure to read the three sections of this Medication Guide. The first section is about the risk of changes in thinking and behavior, depression and suicidal thoughts or actions with medicines used to quit smoking; the second section is about the risk of suicidal thoughts and actions with antidepressant medicines; and the third section is entitled "What Other Important Information Should I Know About BUPROBAN®?"
Quitting Smoking, Quit-Smoking Medications, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions
This section of the Medication Guide is only about the risk of changes in thinking and behavior, depression and suicidal thoughts or actions with drugs used to quit smoking. Talk to your healthcare provider or your family member's healthcare provider about:
- all risks and benefits of quit-smoking medicines.
- all treatment choices for quitting smoking.
Some people have had changes in behavior, hostility, agitation, depression, suicidal thoughts or actions while taking BUPROBAN® to help them quit smoking. These symptoms can develop during treatment with BUPROBAN® or after stopping treatment with BUPROBAN®.
If you, your family member, or your caregiver notices agitation, hostility, depression, or changes in thinking or behavior that are not typical for you, or you have any of the following symptoms, stop taking BUPROBAN® and call your healthcare provider right away:
|
|
When you try to quit smoking, with or without BUPROBAN®, you may have symptoms that may be due to nicotine withdrawal, including urge to smoke, depressed mood, trouble sleeping, irritability, frustration, anger, feeling anxious, difficulty concentrating, restlessness, decreased heart rate, and increased appetite or weight gain. Some people have even experienced suicidal thoughts when trying to quit smoking without medication. Sometimes quitting smoking can lead to worsening of mental health problems that you already have, such as depression.
Before taking BUPROBAN®, tell your healthcare provider if you have ever had depression or other mental illnesses. You should also tell your healthcare provider about any symptoms you had during other times you tried to quit smoking, with or without BUPROBAN®.
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Although BUPROBAN® is not a treatment for depression, it contains bupropion, the same active ingredient as the antidepressant medications WELLBUTRIN®, WELLBUTRIN SR®, and WELLBUTRIN XL®.
This section of the Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines.
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
-
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment. -
2. Depression or other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions. -
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call your healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
|
|
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
It is not known if BUPROBAN® is safe and effective in children under the age of 18.
What other important information should I know about BUPROBAN®?
-
Seizures: There is a chance of having a seizure (convulsion, fit) with BUPROBAN®, especially in people:
- with certain medical problems.
- who take certain medicines.
The chance of having seizures increases with higher doses of BUPROBAN®. For more information, see the sections "Who should not take BUPROBAN®?" and "What should I tell my healthcare provider before taking BUPROBAN®?" Tell your healthcare provider about all of your medical conditions and all the medicines you take. Do not take any other medicines while you are taking BUPROBAN® unless your healthcare provider has said it is okay to take them.
If you have a seizure while taking BUPROBAN®, stop taking the tablets and call your healthcare provider right away. Do not take BUPROBAN® again if you have a seizure.
- High blood pressure (hypertension). Some people get high blood pressure that can be severe, while taking BUPROBAN®. The chance of high blood pressure may be higher if you also use nicotine replacement therapy (such as a nicotine patch) to help you stop smoking (see the section of this Medication Guide called "How should I take BUPROBAN®?").
-
Manic episodes. Some people may have periods of mania while taking BUPROBAN®, including:
- Greatly increased energy
- Severe trouble sleeping
- Racing thoughts
- Reckless behavior
- Unusually grand ideas
- Excessive happiness or irritability
- Talking more or faster than usual
If you have any of the above symptoms of mania, call your healthcare provider.
- Unusual thoughts or behaviors. Some patients have unusual thoughts or behaviors while taking BUPROBAN®, including delusions (believe you are someone else), hallucinations (seeing or hearing things that are not there), paranoia (feeling that people are against you), or feeling confused. If this happens to you, call your healthcare provider.
- Severe allergic reactions. Some people can have severe allergic reactions to BUPROBAN®. Stop taking BUPROBAN® and call your healthcare provider right away if you get a rash, itching, hives, fever, swollen lymph glands, painful sores in the mouth or around the eyes, swelling of the lips or tongue, chest pain, or have trouble breathing. These could be signs of a serious allergic reaction.
What is BUPROBAN®?
BUPROBAN® is a prescription medicine to help people quit smoking.
BUPROBAN® should be used with a patient support program. It is important to participate in the behavioral program, counseling, or other support program your healthcare professional recommends.
Quitting smoking can lower your chances of having lung disease, heart disease, or getting certain types of cancer that are related to smoking.
Who should not take BUPROBAN®?
Do not take BUPROBAN® if you:
- have or had a seizure disorder or epilepsy.
- have or had an eating disorder such as anorexia nervosa or bulimia.
- are taking any other medicines that contain bupropion, including WELLBUTRIN®, WELLBUTRIN SR®, WELLBUTRIN XL®, APLENZIN®, or FORFIVO XL™. Bupropion is the same active ingredient that is in BUPROBAN®.
- drink a lot of alcohol and abruptly stop drinking, or take medicines called sedatives (these make you sleepy), benzodiazepines, or anti-seizure medicines, and you stop taking them all of a sudden.
- take a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- do not take an MAOI within 2 weeks of stopping BUPROBAN® unless directed to do so by your healthcare provider.
- do not start BUPROBAN® if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your healthcare provider.
- are allergic to the active ingredient in BUPROBAN®, bupropion, or to any of the inactive ingredients. See the end of this Medication Guide for a complete list of ingredients in BUPROBAN®.
What should I tell my healthcare provider before taking BUPROBAN®?
Tell your healthcare provider if you have ever had depression, suicidal thoughts or actions, or other mental health problems. You should also tell your healthcare provider about any symptoms you had during other times you tried to quit smoking, with or without BUPROBAN®. See "Quitting Smoking, Quit-Smoking Medications, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions."
-
Tell your healthcare provider about your other medical conditions, including if you:
- have liver problems, especially cirrhosis of the liver.
- have kidney problems.
- have, or have had, an eating disorder such as anorexia nervosa or bulimia.
- have had a head injury.
- have had a seizure (convulsion, fit).
- have a tumor in your nervous system (brain or spine).
- have had a heart attack, heart problems, or high blood pressure.
- are a diabetic taking insulin or other medicines to control your blood sugar.
- drink alcohol.
- abuse prescription medicines or street drugs.
- are pregnant or plan to become pregnant.
- are breastfeeding. BUPROBAN® passes into your milk in small amounts.
- Tell your healthcare provider about all the medicines you take, including prescription, over-the-counter medicines, vitamins, and herbal supplements. Many medicines increase your chances of having seizures or other serious side effects if you take them while you are taking BUPROBAN®.
How should I take BUPROBAN®?
- Start BUPROBAN® before you stop smoking to give BUPROBAN® time to build up in your body. It takes about 1 week for BUPROBAN® to start working.
- Pick a date to stop smoking that is during the second week you are taking BUPROBAN®.
- Take BUPROBAN® exactly as prescribed by your healthcare provider. Do not change your dose or stop taking BUPROBAN® without talking with your healthcare provider first.
- BUPROBAN® is usually taken for 7 to 12 weeks. Your healthcare provider may decide to prescribe BUPROBAN® for longer than 12 weeks to help you stop smoking. Follow your healthcare provider's instructions.
- Swallow BUPROBAN® Tablets whole. Do not chew, cut, or crush BUPROBAN® Tablets. If you do, the medicine will be released into your body too quickly. If this happens you may be more likely to get side effects including seizures. Tell your healthcare provider if you cannot swallow tablets.
- BUPROBAN® Tablets may have an odor. This is normal.
- Take your doses of BUPROBAN® at least 8 hours apart.
- You may take BUPROBAN® with or without food.
- It is not dangerous to smoke and take BUPROBAN® at the same time. But, you will lower your chance of breaking your smoking habit if you smoke after the date you set to stop smoking.
- You may use BUPROBAN® and nicotine patches (a type of nicotine replacement therapy) at the same time, following the precautions below.
- You should only use BUPROBAN® and nicotine patches together under the care of your healthcare provider. Using BUPROBAN® and nicotine patches together may raise your blood pressure, and sometimes this can be severe.
- Tell your healthcare provider if you plan to use nicotine patches. Your healthcare provider should check your blood pressure regularly if you use nicotine patches with BUPROBAN® to help you quit smoking.
- If you miss a dose, do not take an extra dose to make up for the dose you missed. Wait and take your next dose at the regular time. This is very important. Too much BUPROBAN® can increase your chance of having a seizure.
- If you take too much BUPROBAN®, or overdose, call your local emergency room or poison control center right away.
Do not take any other medicines while taking BUPROBAN® unless your healthcare provider has told you it is okay.
What should I avoid while taking BUPROBAN®?
- Limit or avoid using alcohol during treatment with BUPROBAN®. If you usually drink a lot of alcohol, talk with your healthcare provider before suddenly stopping. If you suddenly stop drinking alcohol, you may increase your chance of having seizures.
- Do not drive a car or use heavy machinery until you know how BUPROBAN® affects you. BUPROBAN® can affect your ability to do these things safely.
What are possible side effects of BUPROBAN®?
BUPROBAN® can cause serious side effects. See the sections at the beginning of this Medication Guide for information about serious side effects of BUPROBAN®.
The most common side effects of BUPROBAN® include:
- trouble sleeping
- stuffy nose
- dry mouth
- dizziness
- feeling anxious
- nausea
- constipation
- joint aches
If you have trouble sleeping, do not take BUPROBAN® too close to bedtime.
Tell your healthcare provider right away about any side effects that bother you.
These are not all the possible side effects of BUPROBAN®. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to TEVA USA, PHARMACOVIGILANCE at 1-866-832-8537.
How should I store BUPROBAN®?
- Store BUPROBAN® at room temperature, 20°C to 25°C (68°F to 77°F).
- Keep BUPROBAN® dry and out of the light.
Keep BUPROBAN® and all medicines out of the reach of children.
General information about BUPROBAN®
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BUPROBAN® for a condition for which it was not prescribed.
Do not give BUPROBAN® to other people, even if they have the same symptoms you have. It may harm them.
If you take a urine drug screening test, BUPROBAN® may make the test result positive for amphetamines. If you tell the person giving you the drug screening test that you are taking BUPROBAN®, they can do a more specific drug screening test that should not have this problem.
This Medication Guide summarizes important information about BUPROBAN®. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about BUPROBAN® that is written for health professionals.
For more information about BUPROBAN®, call 1-888-838-2872.
What are the ingredients in BUPROBAN®?
Active ingredient: bupropion hydrochloride.
Inactive ingredients: colloidal silicon dioxide, hydroxypropylcellulose, hypromellose, iron oxide yellow, macrogol, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide and triacetin.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
WELLBUTRIN®, WELLBUTRIN SR®, and WELLBUTRIN XL® are registered trademarks of the GlaxoSmithKline group of companies.
FORFIVO XL™ is a registered trademark of Edgemont Pharmaceuticals, LLC.
APLENZIN® is a registered trademark of sanofi-aventis U.S. LLC.
Manufactured by:
Impax Laboratories, Inc.
Hayward, CA 94544 USA
Manufactured for:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
530-08
Rev. 04/2014
PRINCIPAL DISPLAY PANEL - 150 mg Bottle Label
NDC 0093-5703-01
BUPROBAN
®
[buPROPion HCl
Extended-release Tablets (SR)]
150 mg
Twice-A-Day*
WARNING: Do not use in combination with
Wellbutrin®, Wellbutrin SR®, Wellbutrin XL®, or any
other medicines that contain bupropion hydrochloride.
DISPENSE ATTACHED MED GUIDE
Rx only
100 TABLETS
TEVA
BUPROBANBUPROPION HYDROCHLORIDE TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||