BreathAway
BreathAway Mouth Rinse Cinnamon Content of Label
FULL PRESCRIBING INFORMATION
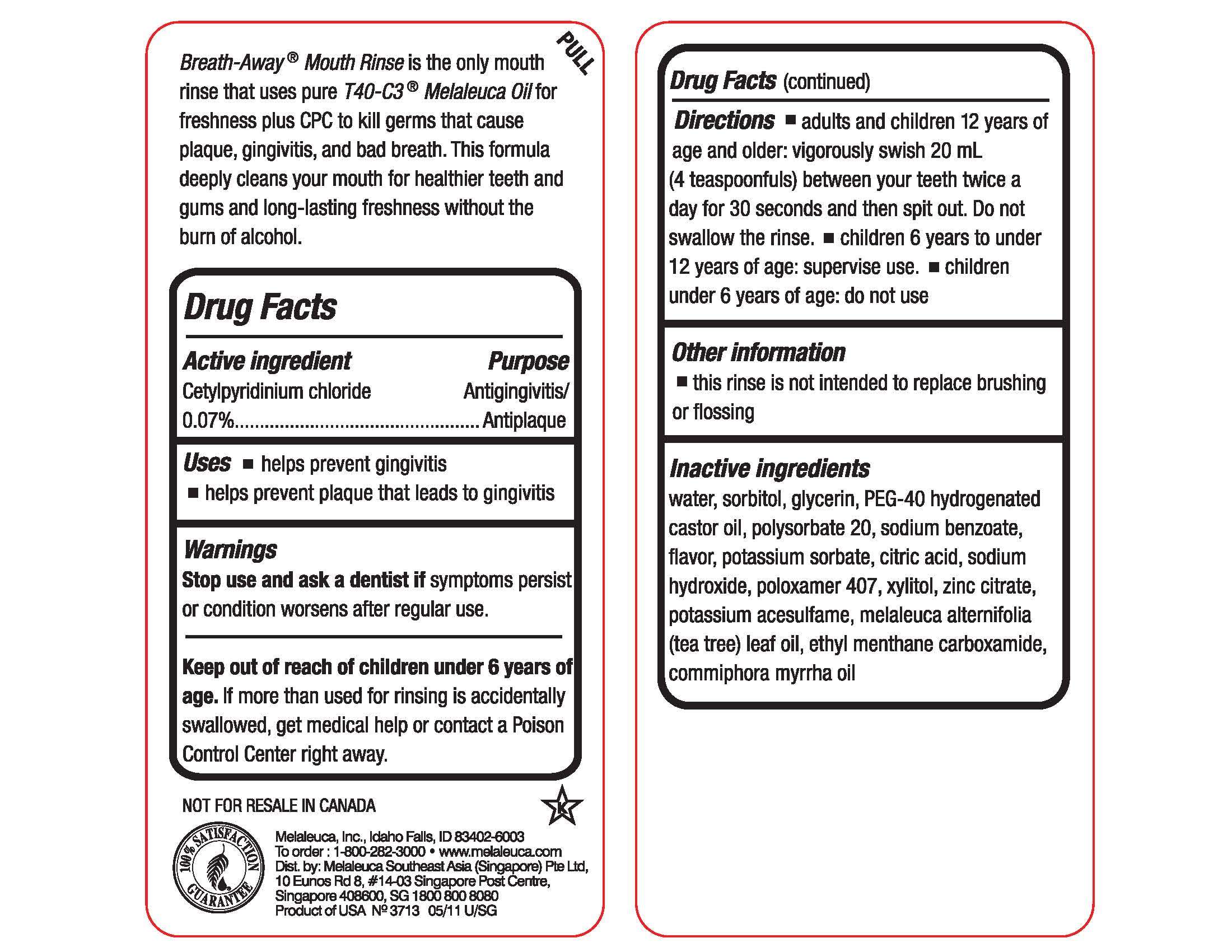
Active ingredient
Active ingredient
Cetylpyridinium chloride 0.07%
Purpose
Purpose
Antigingivitis/Antiplaque
Uses
Use
- helps prevent gingivitis
- helps prevent plaque that leads to gingivitis
Warnings
Stop use and ask a dentist if symptoms persist or condition worsens after regular use.
Keep out of reach of children under 6 years of age. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12years of age and older: vigorously swish 20 mL (4 teaspoonfuls) between your teeth twice a day for 30 seconds and then spit out. Do not swallow the rinse.
- children 6 years to under 12 years of age: supervise use.
- children under 6 years of age: do not use
Inactive ingredients
water, sorbitol, glycerin. PEG-40 hydrogenated castor oil, polysorbate 20, sodium benzoate, flavor, potassium sorbate, citric acid, sodium hydroxide, poloxamer 407, xylitol, zinc citrate, potassium acesulfame, melaleuca alternifolia (tea tree) leaf oil, ethyl menthane carboxamide, commiphora myrrha oil

BreathAwayCetylpyridinium Chloride MOUTHWASH
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||