Blue Gel
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Blue Gel Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Keep Out of Reach of Children
- Directions
- Blue Gel Other information
- Inactive ingredients
- Questions or comments?
- Additional Information Listed on Other Panels
- Principal Display
FULL PRESCRIBING INFORMATION
Active ingredient
Menthol 2%
Purpose
Analgesic
Blue Gel Uses
● temporarily relieves minor aches and pains of muscles and joints associated with:
● arthritis ● simple backache ● strains ● sprains
● provides cooling penetrating relief
Warnings
For external use only
Do not use
- on wounds or damaged skin
When using this product
- avoid contact with the eyes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding
ask a health professional before use
Keep Out of Reach of Children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean and dry skin
- may be used with wet or dry dandages or ice packs
- adults & children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years: ask a doctor
Blue Gel Other information
- store at room temperature
- keep container tightly closed
- keep away from heat or open flame
Inactive ingredients
ammonium hydroxide, carbopol 934P, cupric sulfate monohydrate, FD&C blue no. 1, isopropyl alcohol, magnesium sulfate heptyhydrate, sodium hydroxide, thymol, water
Questions or comments?
call 1-800-645-2158
Additional Information Listed on Other Panels
Distributed by:
Rugby Laboratories
Livonia, MI 48150 USA
Blue Gel
External Analgesic
Pain Relieving Gel
Principal Display
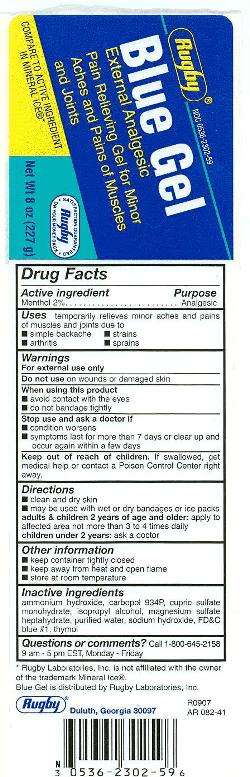
Blue GelMenthol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!