Blistex
Blistex Foille Medicated First Aid Ointment
FULL PRESCRIBING INFORMATION: CONTENTS*
- Blistex Uses
- Warnings
- Directions
- Blistex Other information
- Inactive ingredients
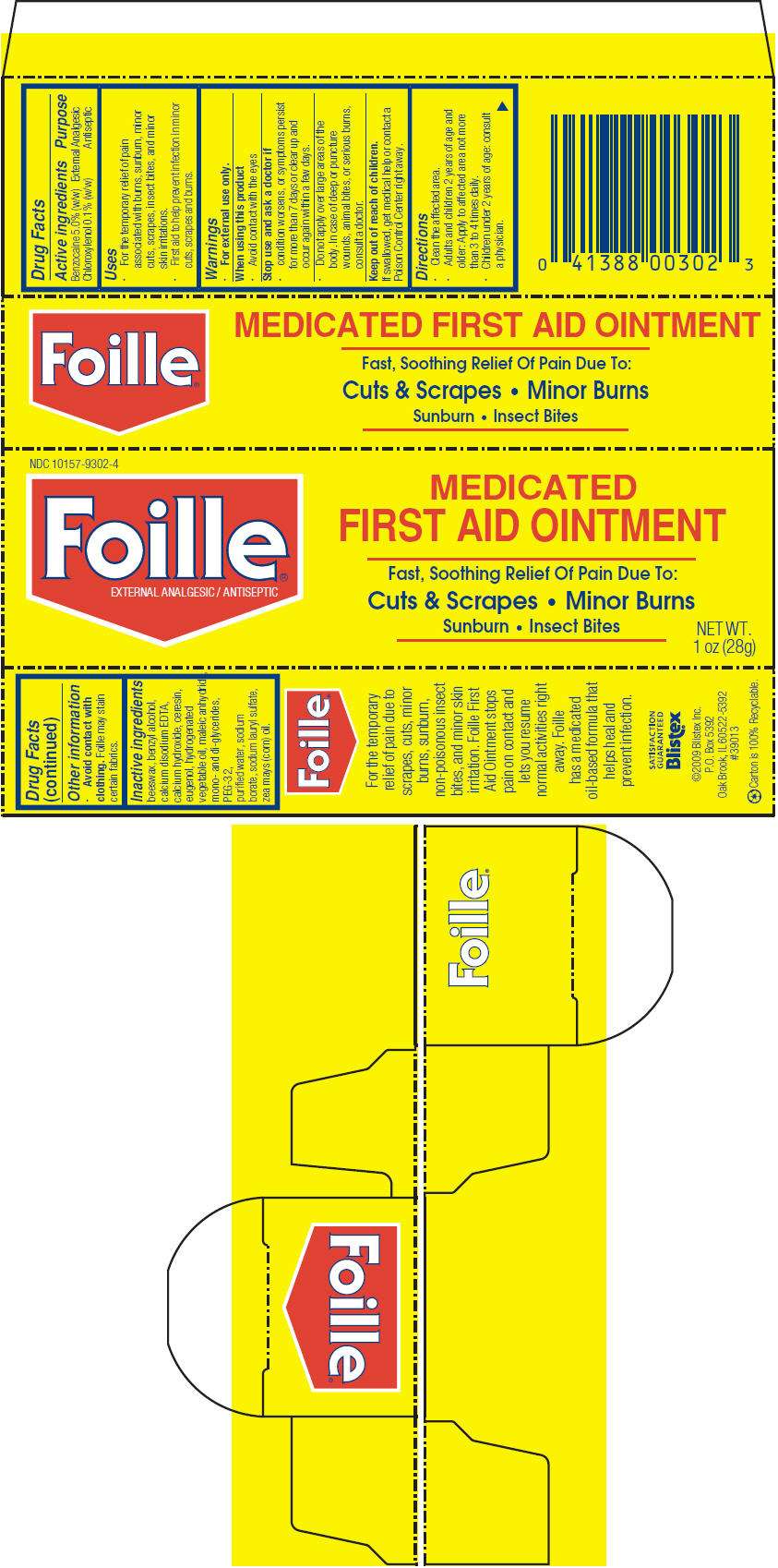
- PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| Benzocaine 5.0% (w/w) | External Analgesic |
| Chloroxylenol 0.1% (w/w) | Antiseptic |
Blistex Uses
- For the temporary relief of pain associated with burns, sunburn, minor cuts, scrapes, insect bites, and minor skin irritations.
- First aid to help prevent infection in minor cuts, scrapes and burns.
Warnings
- For external use only.
When using this product
- Avoid contact with the eyes
Stop use and ask a doctor if
- condition worsens, or symptoms persist for more than 7 days or clear up and occur again within a few days.
- Do not apply over large areas of the body. In case of deep or puncture wounds, animal bites, or serious burns, consult a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Clean the affected area.
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 2 years of age: consult a physician.
Blistex Other information
- Avoid contact with clothing. Foille may stain certain fabrics.
Inactive ingredients
beeswax, benzyl alcohol, calcium disodium EDTA, calcium hydroxide, ceresin, eugenol, hydrogenated vegetable oil, maleic anhydride, mono- and di-glycerides, PEG-32, purified water, sodium borate, sodium lauryl sulfate, zea mays (corn) oil.
PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
NDC 10157-9302-4
Foille
®
EXTERNAL ANALGESIC / ANTISEPTIC
MEDICATED
FIRST AID OINTMENT
Fast, Soothing Relief Of Pain Due To:
Cuts & Scrapes • Minor Burns
Sunburn • Insect Bites
NET WT.
1 oz (28g)
BlistexBenzocaine and Chloroxylenol OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||