Pharbest Pharmaceuticals Inc.
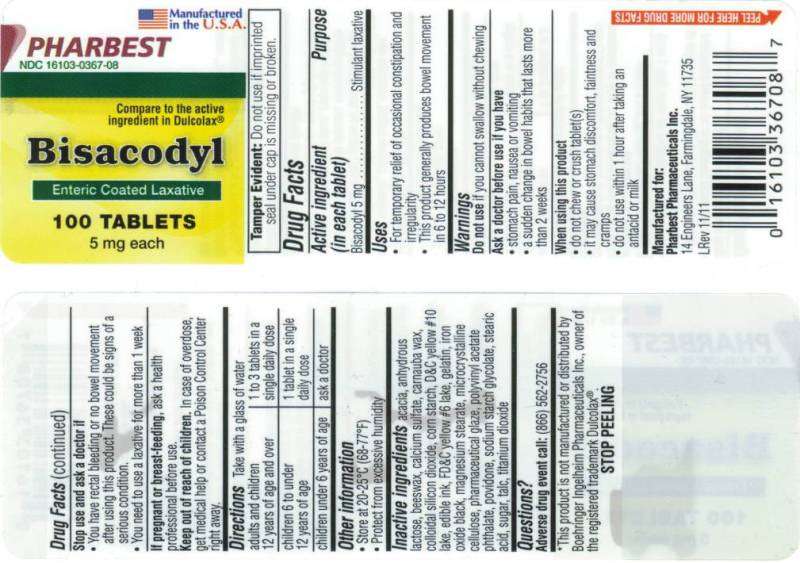
Drug facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Warnings
- Directions
- Bisacodyl Other information
- Inactive ingredients:
acacia, anhydrous lactose, beeswax, calcium sulfate, carnauba wax, colloidal silicon dioxide, corn starch, D&C yellow #10 lake, edible ink, FD&C yellow #6 lake, gelatin, iron oxide black, magnesium stearate, microcrystalline cellulos
- Questions?
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Purpose
Uses
- For temporary relief of occasional constipation and irregularity
- This product generally produces bowel movement in 6 to 12 hours
Warnings
Do not use
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product
- do not chew or crush tablet(s)
- it may cause stomach discomfort, faintness and cramps
- do not use within 1 hour after taking an antacid or milk
Stop use and ask a doctor if
- You have rectal bleeding or no bowel movement after using this product. These could be signs of a serious condition.
- You need to use a laxative for more than 1 week
If pregnant or breast-feeding,
Keep out of reach of children.
Directions
adults and children 12 years of age and over
|
1 to 3 tablets in a single daily dose
|
children 6 to under 12 years of age
|
1 tablet in a single daily dose
|
children under 6 years of age
|
ask a doctor
|
Bisacodyl Other information
- Store at 20 - 250C (68-770F)
- Protect from excessive humidity
Inactive ingredients:
acacia, anhydrous lactose, beeswax, calcium sulfate, carnauba wax, colloidal silicon dioxide, corn starch, D&C yellow #10 lake, edible ink, FD&C yellow #6 lake, gelatin, iron oxide black, magnesium stearate, microcrystalline cellulos
Questions?
Adverse drug event call: (866) 562-2756
Bisacodyl
Bisacodyl TABLET, DELAYED RELEASE
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:16103-367 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
Bisacodyl Bisacodyl |
|
5 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
orange |
6 mm |
S1 |
ROUND |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:16103-367-17 |
25 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:16103-367-08 |
100 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part334 |
2006-01-25 |
|
|
