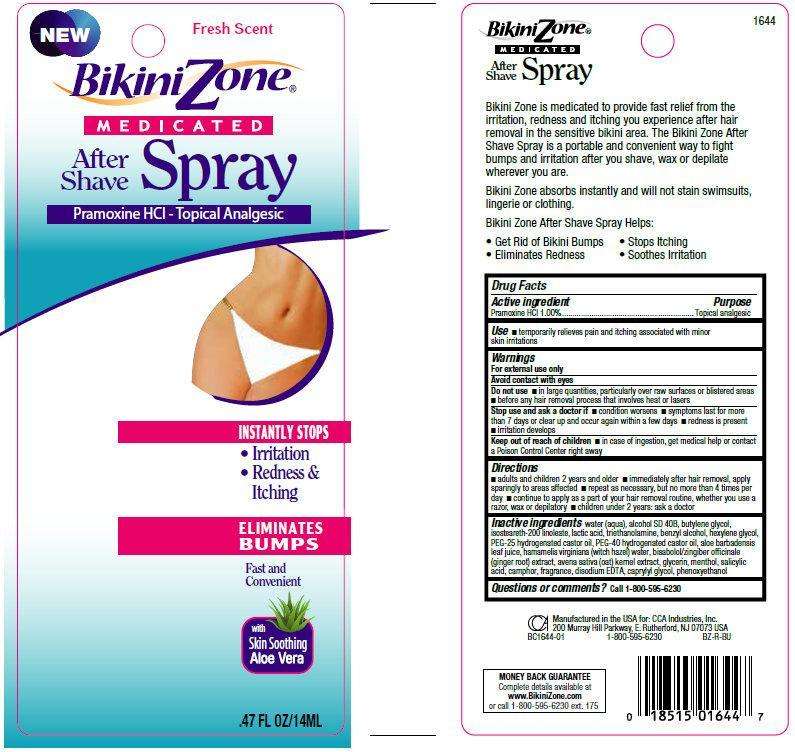
Bikini Zone MEDICATED After Shave Fresh Scent
Bikini Zone MEDICATED After Shave Spray Fresh Scent
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions or comments?
- Products Labels
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Pramoxine HCI 1.00%
Purpose
Topical analgesic
Use
- temporarily relieves pain and itching associated with minor skin irritations
Warnings
- For external use only.
Avoid
contact with eyes
Do not use
- in large quantities, particularly over raw surfaces or blistered areas
- before any hair removal process that involves heat or lasers
Stop use and ask a doctor if
- condition worsens
- symptoms last for more than 7 days or clear up and occur again within a few days
- redness is present
- irritation develops
Keep out of reach of children
- in case of ingestion, get medical help or contact a Poison Control Center right away
Directions
- adults and children 2 years and older
- immediately after hair removal, apply sparingly to areas affected
- repeat as necessary, but no more than 4 times per day
- continue to apply as a part of your hair removal routine, whether you use a razor, wax or depilatory
- children under 2 years: ask a doctor
Inactive ingredients
water (aqua), alcohol SD 40B, butylene glycol, isosteareth-200 linoleate, lactic acid, triethanolamine, benzyl alcohol, hexylene glycol, PEG-25 hydrogenated castor oil, PEG-40 hydrogenated castor oil, aloe barbadensis leaf juice, hamamelis virginiana (witch hazel) water, bisabolol/zingiber officinale (ginger root) extract, avena sativa (oat) kernel extract, glycerin, menthol, salicylic acid, camphor, fragrance, disodium EDTA, caprylyl glycol, phenoxyethanol
Questions or comments?
- Call 1-800-595-6230
Products Labels


Bikini Zone MEDICATED After Shave Fresh ScentPRAMOXINE HYDROCHLORIDE SPRAY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!