Benztropine Mesylate
FULL PRESCRIBING INFORMATION: CONTENTS*
- BENZTROPINE MESYLATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- BENZTROPINE MESYLATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- BENZTROPINE MESYLATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BENZTROPINE MESYLATE DESCRIPTION
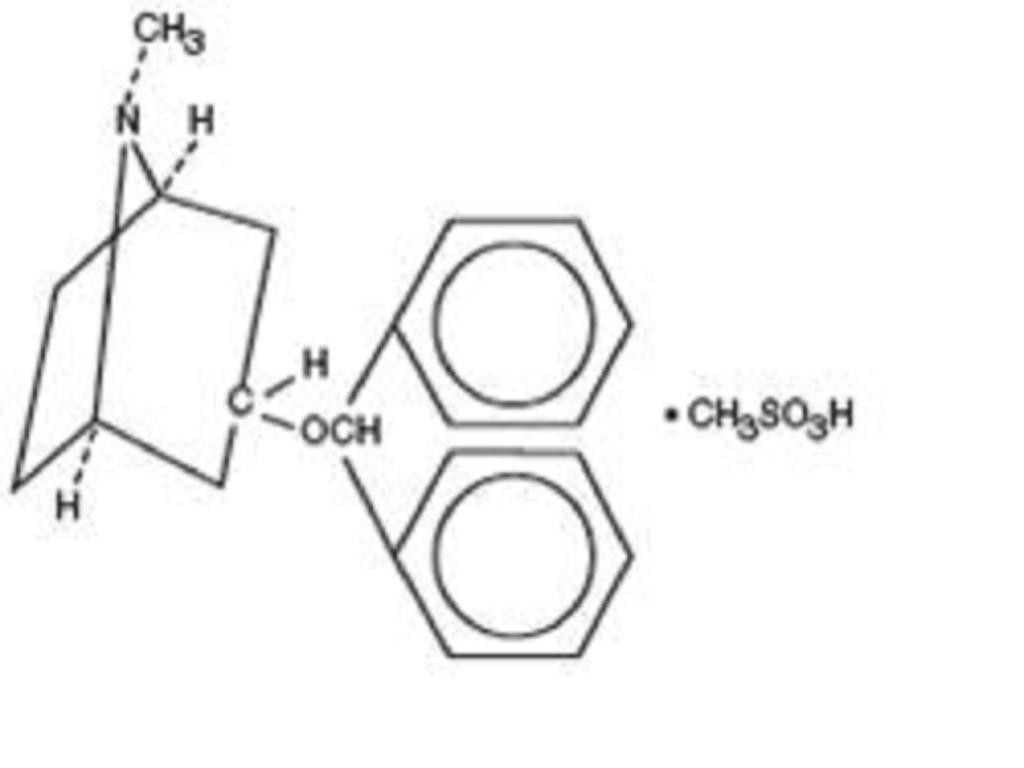
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
PRECAUTIONS
BENZTROPINE MESYLATE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralWARNINGS
CONTRAINDICATIONS
BENZTROPINE MESYLATE ADVERSE REACTIONS
OVERDOSAGE
Manifestations
Treatment
DOSAGE & ADMINISTRATION
Postencephalitic and Idiopathic Parkinsonism
Drug-Induced Extrapyramidal Disorders
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Benztropine MesylateBenztropine Mesylate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!