BELLA SCHNEIDER BEAUTY Glow And Go Tinted Moisturizing Sunscreen Broad Spectrum SPF 30
BELLA SCHNEIDER BEAUTY Glow And Go Tinted Moisturizing Sunscreen Broad Spectrum SPF 30
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Active ingredients
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Questions or comments?
- GLOW & GO TINTED MOISTURIZING SUNSCREEN
- Product Labels
FULL PRESCRIBING INFORMATION
Drug Facts
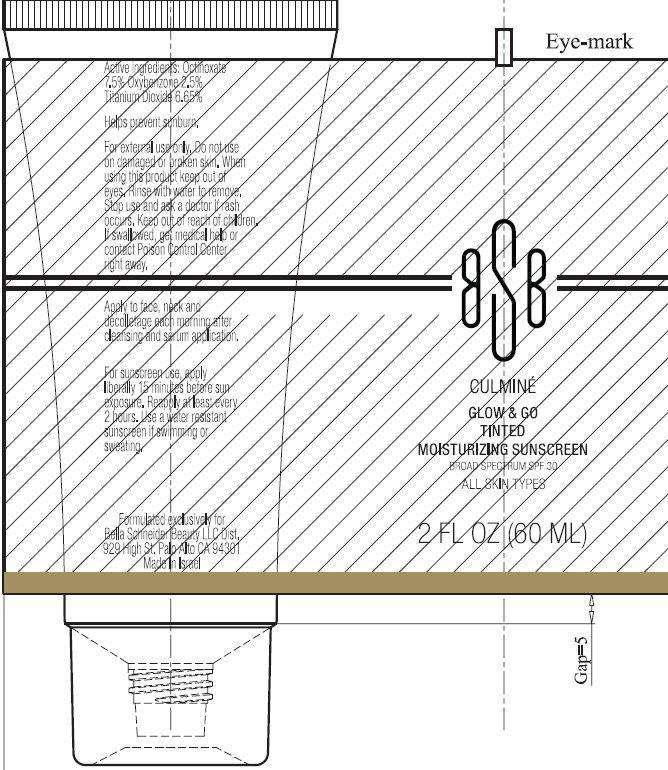
Active ingredients
Octinoxate 7.5%
Oxybenzone 2.5%
Titanium Dioxide 6.65%
Purpose
Sunscreen
Use
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not use
- on damaged or broken skin
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor
- if rash occurs
Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
For sunscreen use
■ Apply liberally 15 minutes before sun exposure
■ Reapply at least every 2 hours
■ Use a water resistant sunscreen if swimming or sweating
■ Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
■ Limit time in the sun, especially from 10 a.m. – 2 p.m.
■ Wear long-sleeved shirts, pants, hats and sunglasses
■ Children under 6 months of age: Ask a doctor
Other Information
■ Protect the product in this container from excessive heat and direct sun.
Inactive ingredients
WATER, ALKYL BENZOATE, GLYCERIN, CAPRYLIC/CAPRIC TRIGLYCERIDE, PENTAERYTHRITYL DISTEARATE, PROPANEDIOL, JOJOBA (SIMMONDSIA CHINENSIS) SEED OIL, GLYCERYL STEARATE, TEA (CAMELLIA SINENSIS) LEAF EXTRACT, CARRAGEENAN (CHONDRUS CRISPUS), PANTHENOL, SODIUM STEAROYL GLUTAMATE, TOCOPHERYL ACETATE, SCLEROTIUM GUM, DIMETHICONE, PHENOXYETHANOL, XANTHAN GUM, SODIUM POLYACRYLATE, BUTYLENE GLYCOL, CAPRYLYL GLYCOL, TOCOPHEROL, FRAGRANCE, CHLORPHENESIN, BETA-SITOSTEROL, SQUALENE, DISODIUM EDTA, IRON OXIDES
Questions or comments?
Call toll-free 1-888-200-3977
GLOW & GO TINTED MOISTURIZING SUNSCREEN
For flawless-looking skin, our antioxidant-infused moisturizer with daytime sun protection delivers the whole package: superior hydration, sheer enough to wear under makeup and a hint of tint that lends bare skin a beautiful glow. Used daily, Glow and Go helps protect against damage, the appearance of fine lines and wrinkles, and discoloration caused by the sun when used with other protective measures (see Drug Facts Chart). Formulated exclusively for Bella Schneider Beauty LLC Dist. 929 High St. Palo Alto, CA 94301 Made In Israel
BELLA SCHNEIDER BEAUTY CULMINE GLOW AND GO TINTED MOISTURIZING SUNSCREEN BROAD SPECTRUM SPF30 ALL SKIN TYPES 2FL OZ (60 ML)
Product Labels

BELLA SCHNEIDER BEAUTY Glow And Go Tinted Moisturizing Sunscreen Broad Spectrum SPF 30OCTINOXATE, OXYBENZONE, TITANIUM DIOXIDE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||