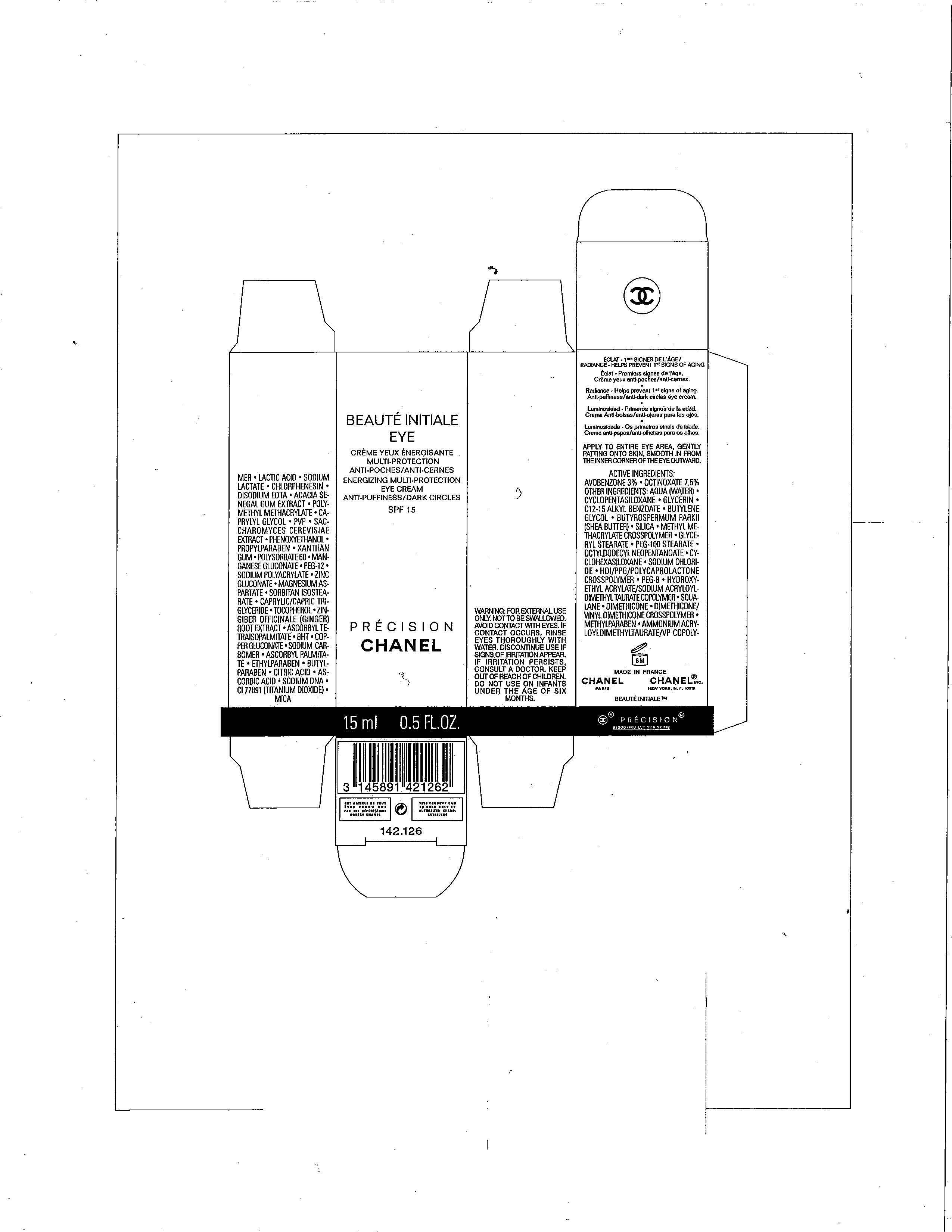
BEAUTE INITIALE
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT(S)
AVOBENZONE 3%
OCTINOXATE 7.5%
WARNINGS
FOR EXTERNAL USE ONLY. NOT TO BE SWALLOWED. AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER, DISCONTINUE USE IF SIGNS OF IRRITATION APPEAR. IF IRRITATION PERSISTS, CONSULT A DOCTOR. KEEP OUT OF REACH OF CHILDREN. DO NOT USE ON INFANTS UNDER THE AGE OF SIX MONTHS.
DIRECTIONS FOR USE
APPLY TO ENTIRE EYE AREA, GENTLY PATTING ONTO SKIN. SMOOTH IN FROM THE INNER CORNER OF THE EYE OUTWARD.
INACTIVE INGREDIENTS
AQUA (WATER), CYCLOPENTASILOXANE, GLYCERIN, C12-15 ALKYL BENZOATE, BUTYLENE GLYCOL, BUTYROSPERMUM PARK II (SHEA BUTTER), SILICA, METHYL METHACRYLATE CROSSPOLYMER, GLYCERYL STEARATE, PEG-100 STEARATE, OCTYLDECYL NEOPENTANOATE, CYCLOHEXASILOXANE, SODIUM CHLORIDE, HDI/PPG/POLYCAPROLACTONE CROSSPOLYMER, PEG-8, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, SQUALANE, DIMETHICONE, DIMETHICONE/VINYL DIMETHICONE COPOLYMER, METHYLPARABEN, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, LACTIC ACID, SODIUM LACTATE, CHLORPHENESIN, DISODIUM EDTA, ACACIA SENEGAL GUM EXTRACT, POLYMETHYL METHACRYLATE, CAPRYLYL GLYCOL, PVP, SACCHROMYCES CEREVISIAE EXTRACT, PHENOXYETHANOL, PROPYLPARABEN, XANTHAN GUM, POLYSORBATE 60, MANGANESE GLUCONATE, PEG-12, SODIUM POLYACRYLATE, ZINC GLUCONATE, PEG-12, SODIUM POLYACRYLATE, ZINC GLUCONATE, MAGNESIUM ASPARTATE, SORBITAN ISOSTEARATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, TOCOPHEROL, ZINGIBER OFFICINALE (GINGER) ROOT EXTRACT, ASCORBYL TETRAISOPALMITATE, BHT, COPPER GLUCONATE, SODIUM CARBOMER, ASCORBYL PALMITATE, ETHYLPARABEN, BUTYLPARABEN, CITRIC ACID, ASCORBIC ACID, SODIUM DNA, CI 77891 (TITANIUM DIOXIDE), MICA
PRINCIPAL DISPLAY PANEL
BEAUTE INITIALE
EYE
ENERGIZING MULTI-PROTECTION
EYE CREAM
ANTI-PUFFINESS/DARK CIRCLES
SPF 15
PRECISION
CHANEL
15 ML 0.5 FL.OZ.
BEAUTE INITIALEEnergizing Multi-Protection Eye Cream CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||