BB Cream Medium
Janssen Cosmetics GmbH
Janssen Cosmetics GmbH
BB Cream Medium
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients: Titanium Dioxide 11.4% Purpose: Sunscreen
Cosmetics Ingredients
Cosmetics Ingredients: small-capssmall-capsWater, Aloe Barbadensis Leaf Juice, Caprylic/Capric Triglyceride, Glycerin Octyldodecyl Stearoyl Stearate, Diisoproply Sebacate, Cetearyl Alcohol, Glyceryl Sterate, Silica, Polyhydroxystearic Acid, Potassium Palmitoyl Hydrolyzed Wheat Protein, CI 77 492 [Iron Oxides],Butyrospermum Parkii Butter, Benzyl Alcohol, Microcrystalline Cellulose, Cetyl Alcohol, Alumina, Stearic Acid, Tocopherol, Citric Acid, Hydrolyzed Beta Maritima Extract, CI 77 491 [Iron Oxides], Solanmum Lycopersicum Seed Oil, [Solanmum Lycopersicum (Tomato) Seed Oil], Xanthan Gum Lavandula Angustifolia Oil, Helianthus Annuus Seed Oil , [Helianthus Annuus (Sunflower) Seed Oil, Cellulose Gum, CI 77 499 [Iron Oxides], Dehydroacetic Acid, Vaccinium Macrocarpon Seed Oil[Vaccinium Macrocarpon (Cranberry) Seed Oil] , Sodium Citrate, Cellulose, Sorbic Acid, Sodium Hydroxide
Warning
For External use only. Not to be sallowed. Avoid contact with the eyes. If contact occurs rinse eyes. Discontinue use if signs of irritation or rash appear. If irriation or rash persists, consult a doctor
Directions
Directions: Simply distribute over the skin for a natural finish
Principal Display Panel
BB Cream Medium
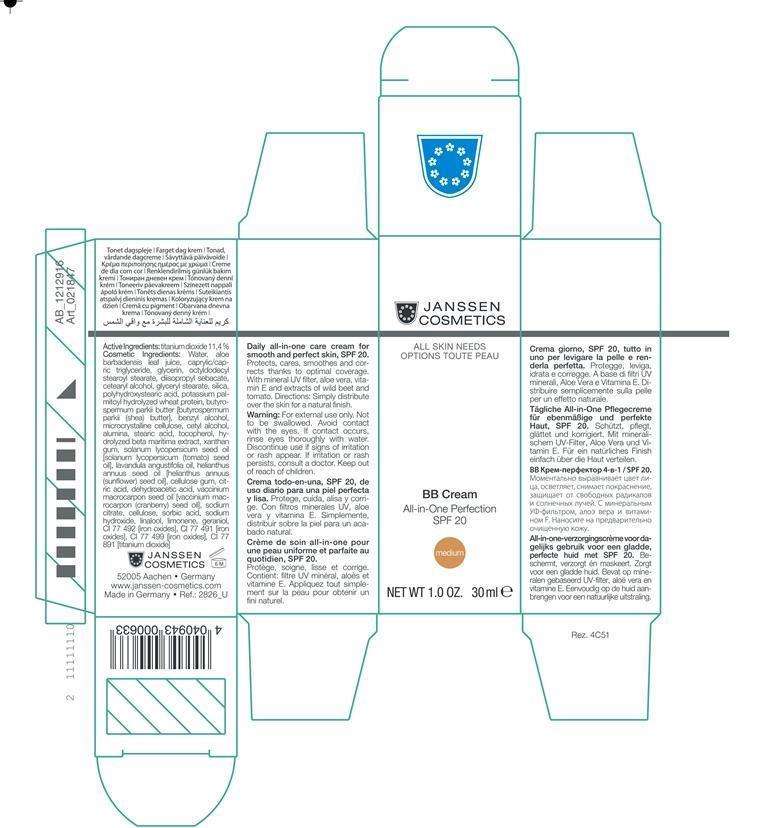
BB Cream MediumTitanium Dioxide CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||