Bare Escentuals Beauty Inc.
BareMinerals SPF 20 Correcting Concealer
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
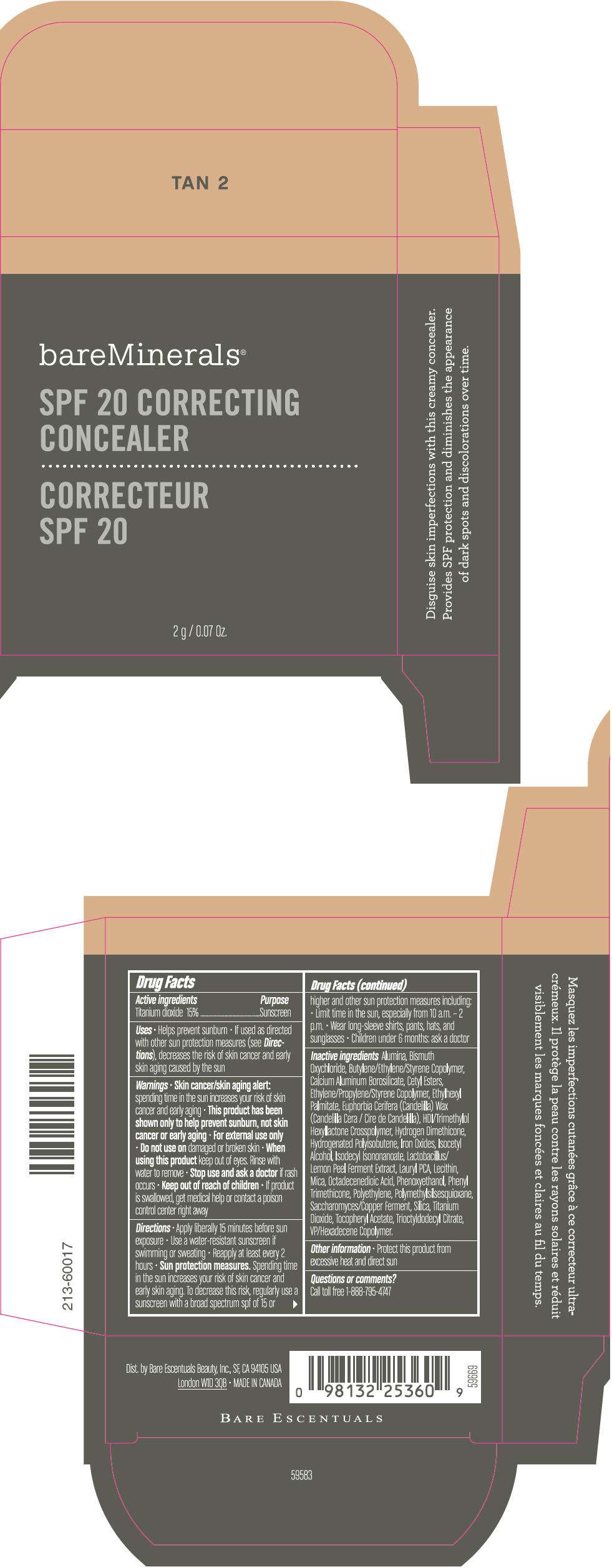
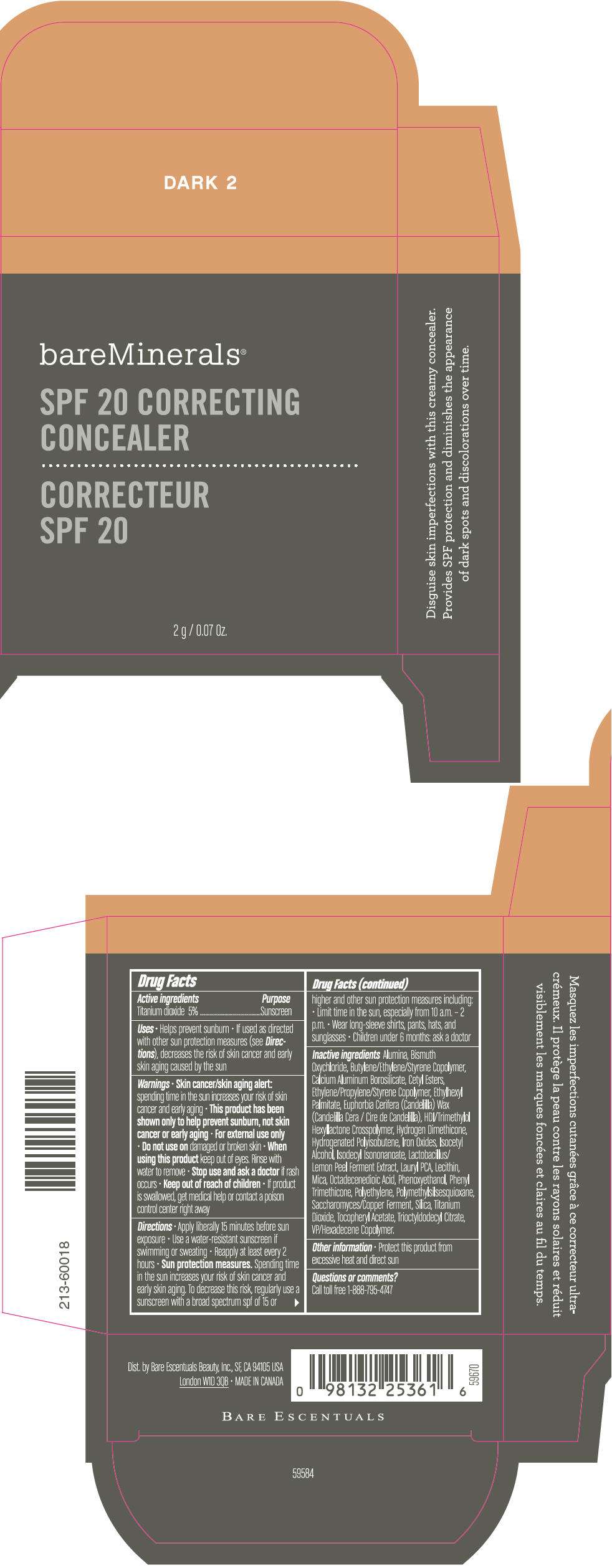
Drug Facts
Active ingredients
Titanium dioxide 15%
Purpose
Sunscreen
BareMinerals Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see
Directions
), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
-
Skin cancer/skin aging alert: spending time in the sun increases your risk of skin cancer and early aging
-
This product has been shown only to help prevent sunburn, not skin cancer or early aging
-
For external use only
-
Do not use on damaged or broken skin
-
When using this product keep out of eyes. Rinse with water to remove
-
Stop use and ask a doctor if rash occurs
-
Keep out of reach of children
- If product is swallowed, get medical help or contact a poison control center right away
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water-resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
-
Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum spf of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: ask a doctor
Inactive ingredients
Alumina, Bismuth Oxychloride, Butylene/Ethylene/Styrene Copolymer, Calcium Aluminum Borosilicate, Cetyl Esters, Ethylene/Propylene/Styrene Copolymer, Ethylhexyl Palmitate, Euphorbia Cerifera (Candelilla) Wax (Candelilla Cera / Cire de Candelilla), HDI/Trimethylol Hexyllactone Crosspolymer, Hydrogen Dimethicone, Hydrogenated Polyisobutene, Iron Oxides, Isocetyl Alcohol, Isodecyl Isononanoate, Lactobacillus/Lemon Peel Ferment Extract, Lauryl PCA, Lecithin, Mica, Octadecenedioic Acid, Phenoxyethanol, Phenyl Trimethicone, Polyethylene, Polymethylsilsesquioxane, Saccharomyces/Copper Ferment, Silica, Titanium Dioxide, Tocopheryl Acetate, Trioctyldodecyl Citrate, VP/Hexadecene Copolymer.
BareMinerals Other information
- Protect this product from excessive heat and direct sun
Questions or comments?
Call toll free 1-888-795-4747
Dist. by Bare Escentuals Beauty, Inc., SF, CA 94105 USA
PRINCIPAL DISPLAY PANEL - 2 g Container Carton - LIGHT 1
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.

PRINCIPAL DISPLAY PANEL - 2 g Container Carton - LIGHT 2
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.
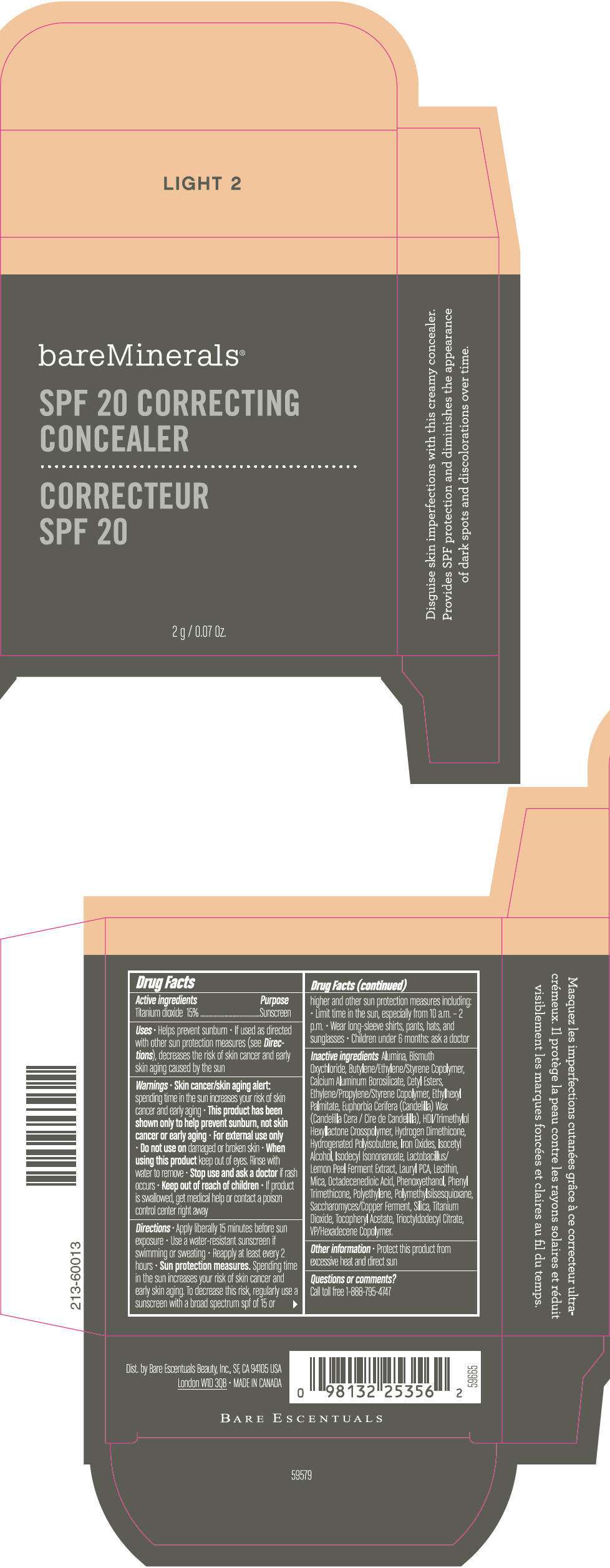
PRINCIPAL DISPLAY PANEL - 2 g Container Carton - MEDIUM 1
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.
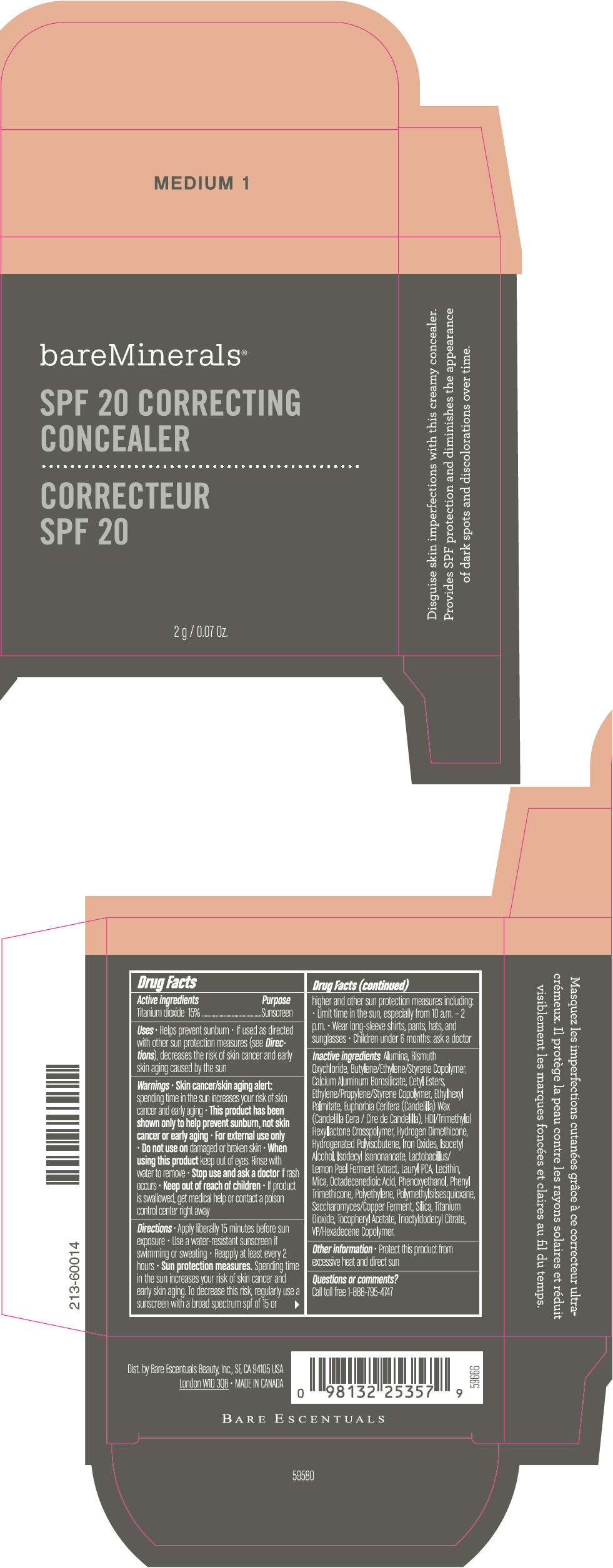
PRINCIPAL DISPLAY PANEL - 2 g Container Carton - MEDIUM 2
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.
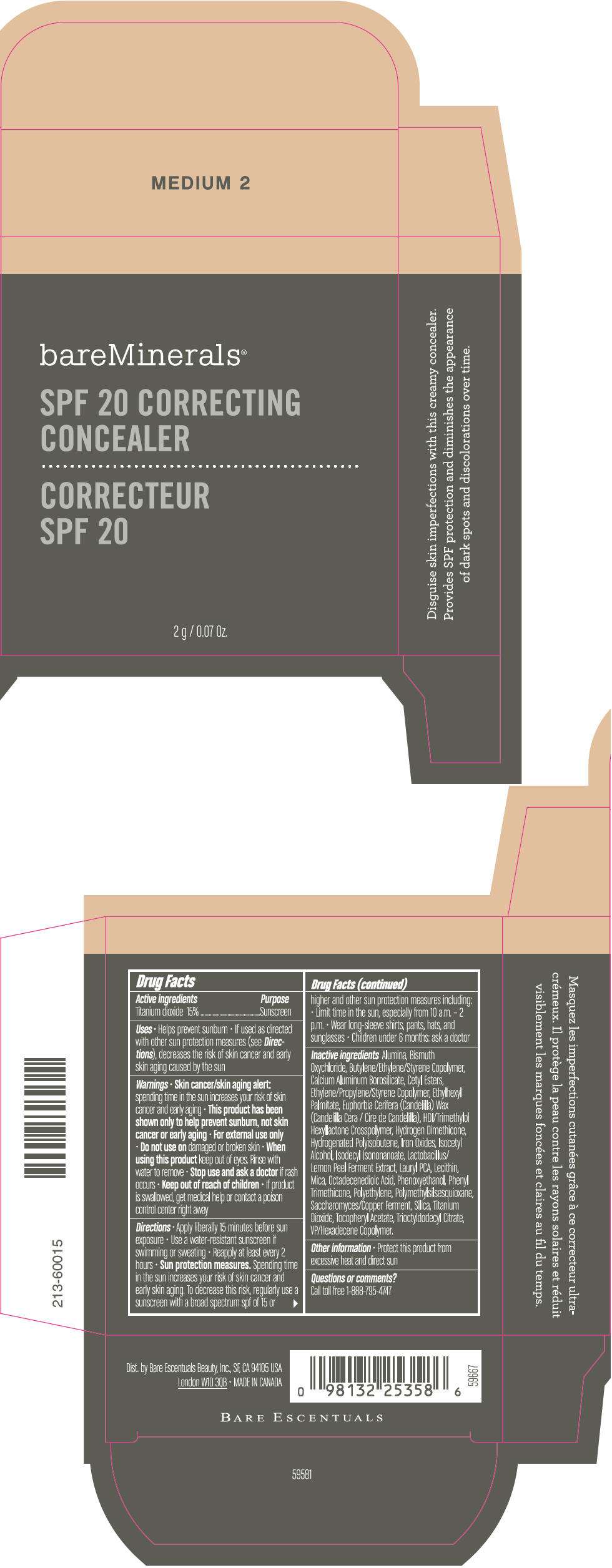
PRINCIPAL DISPLAY PANEL - 2 g Container Carton - TAN 1
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.
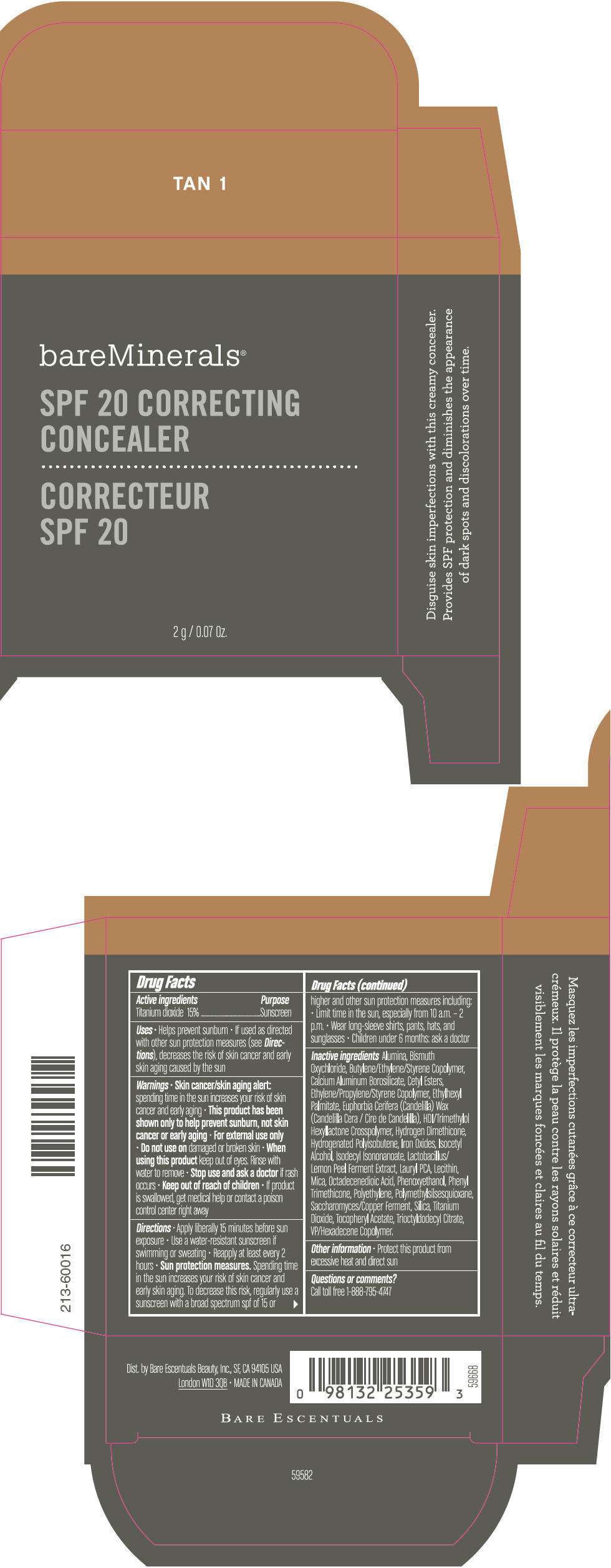
PRINCIPAL DISPLAY PANEL - 2 g Container Carton - TAN 2
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.
PRINCIPAL DISPLAY PANEL - 2 g Container Carton - DARK 2
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.
PRINCIPAL DISPLAY PANEL - 2 g Container Carton - DEEP 2
bareMinerals®
SPF 20 CORRECTING
CONCEALER
2 g / 0.07 Oz.

BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-664 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Light 1) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-664-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|
BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-665 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Light 2) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-665-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|
BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-666 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Medium 1) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-666-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|
BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-667 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Medium 2) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-667-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|
BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-668 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Tan 1) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-668-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|
BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-669 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Tan 2) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-669-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|
BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-670 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Dark 2) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-670-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|
BareMinerals
Titanium Dioxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:98132-671 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
titanium dioxide |
|
0.3 g
|
Product Characteristics
|
|
Color
|
|
BROWN (Deep 2) |
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
2 in 1 CONTAINER |
|
|
|
2 |
NDC:98132-671-01 |
1 in 1 CARTON |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2012-04-26 |
|
|







