Azelastine Hydrochloride
Azelastine Hydrochloride Ophthalmic Solution, 0.05%
FULL PRESCRIBING INFORMATION: CONTENTS*
- AZELASTINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- AZELASTINE HYDROCHLORIDE INDICATIONS AND USAGE
- AZELASTINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AZELASTINE HYDROCHLORIDE ADVERSE REACTIONS
- AZELASTINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON
FULL PRESCRIBING INFORMATION
AZELASTINE HYDROCHLORIDE DESCRIPTION
1

22243
Active:Preservative:Inactives:
CLINICAL PHARMACOLOGY
1
Pharmacokinetics and Metabolism
Clinical Trials
AZELASTINE HYDROCHLORIDE INDICATIONS AND USAGE
AZELASTINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
Information for Patients
whose eyes are not red
Carcinogenesis, Mutagenesis, Impairment of Fertility
µ
Pregnancy
th
Nursing Mothers
Pediatric Use
Geriatric Use
AZELASTINE HYDROCHLORIDE ADVERSE REACTIONS
AZELASTINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
HOW SUPPLIED
Storage
Sun Pharmaceutical Ind. Ltd.
Caraco Pharmaceutical Laboratories, Ltd.
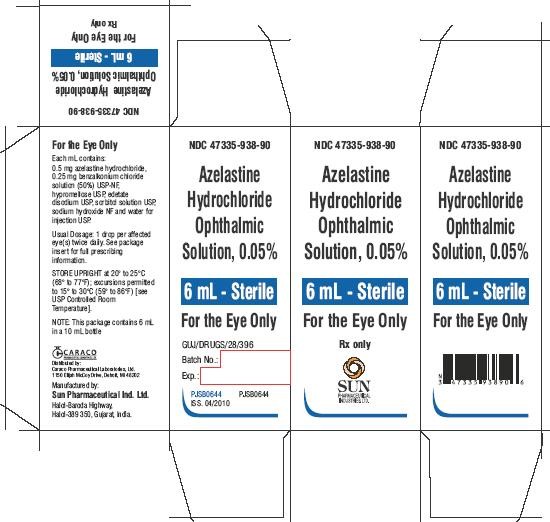
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL
NDC 47335-938-90
Azelastine Hydrochloride Ophthalmic Solution, 0.05%
6 mL - Sterile
For the Eye Only
Rx only

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON
NDC 47335-938-90
Azelastine Hydrochloride Ophthalmic Solution, 0.05%
6 mL - Sterile
For the Eye Only
Rx only
SUN PHARMACEUTICAL INDUSTRIES LTD.
Azelastine HydrochlorideAzelastine Hydrochloride SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!