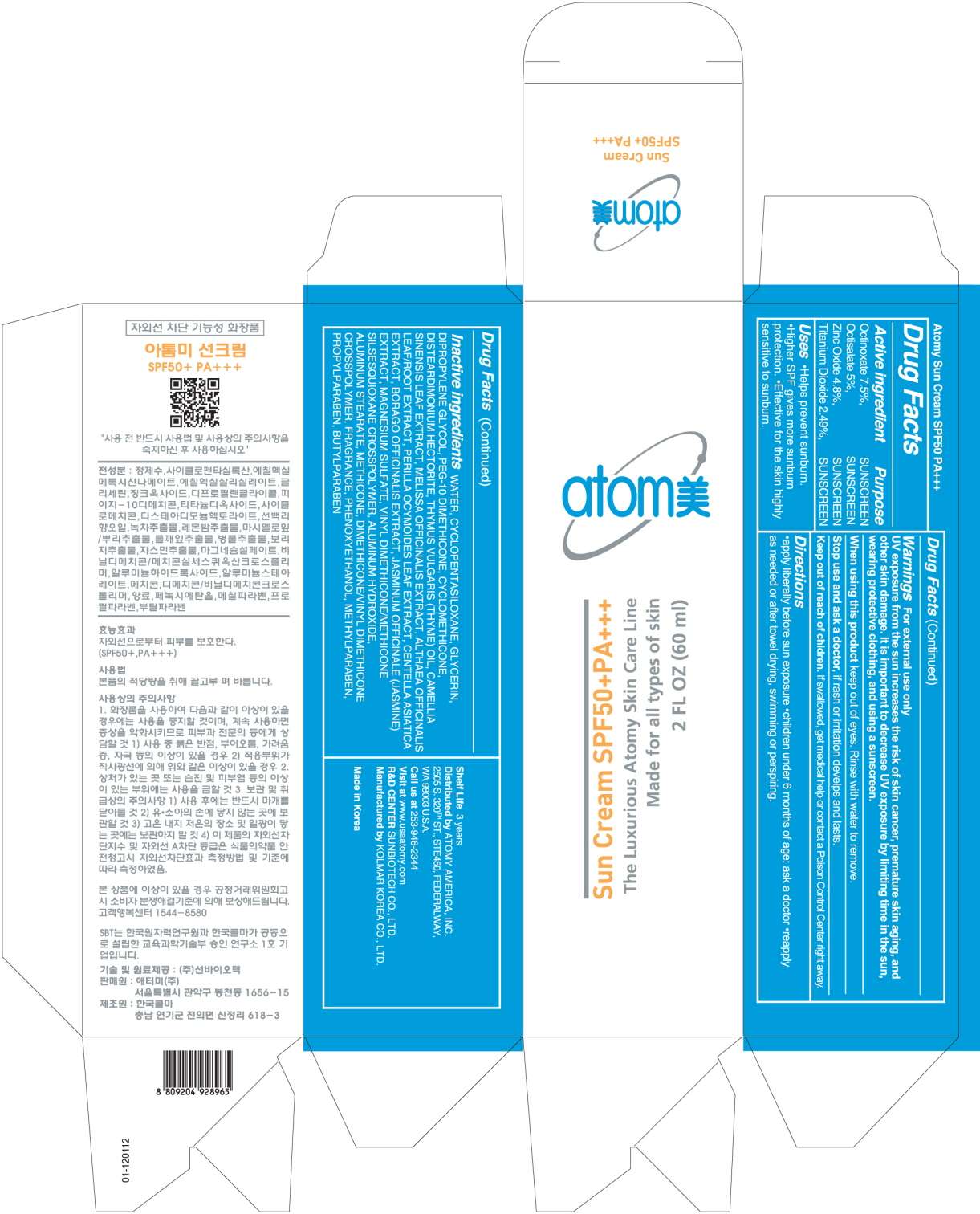
ATOMY SUN
ATOMY CO., LTD.
ATOMY CO., LTD.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients: OCTINOXATE 7.5%, OCTISALATE 5%, ZINC OXIDE 4.8%, TITANIUM DIOXIDE 2.49%
Inactive ingredients:
WATER, CYCLOMETHICONE 5, GLYCERIN, DIPROPYLENE GLYCOL, PEG-10 DIMETHICONE, CYCLOMETHICONE, DISTEARDIMONIUM HECTORITE, MAGNESIUM SULFATE, PHENOXYETHANOL, FRAGRANCE, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, ALUMINUM HYDROXIDE, ALUMINUM STEARATE, METHICONE, METHYLPARABEN, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, PROPYLPARABEN, CAMELLIA SINSENSIS LEAF EXTRACT, THYME OIL, ALTHAEA OFFICINALIS LEAF, MELISSA OFFICINALIS, BORAGO OFFICINALIS EXTRACT, CENTELLA ASIATICA, JASMINUM OFFICINALE FLOWER, PERILLA OCYMOIDES LEAF EXTRACT, BUTYLPARABEN
Purpose
Purpose:
Helps prevent sunburn.
Higher SPF gives more sunburn protection.
Effective for the skin highly sensitive to sunburn.
Warnings:
For external use only.
UV exposure from the sun increases the risk of skin cancer, premature skin aging and other skin damage.
It is important to decrease UV exposure by limiting time in the sun, wearing protective clothing, and using a sunscreen.
When using this product, keep out of eyes. Rinse water to remove.
Stop use and ask a doctor, if rash or irritation develops and lasts.
Keep out of reach of children:
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
Indication and usage:
- Children under 6months of age: ask a doctor.
- Reapply as needed or after towel drying, swimming or perspiring.
Dosage and administration:
Apply liberally before sun exposure.
ATOMY SUNOCTINOXATE, OCTISALATE, ZINC OXIDE, TITANIUM DIOXIDE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||