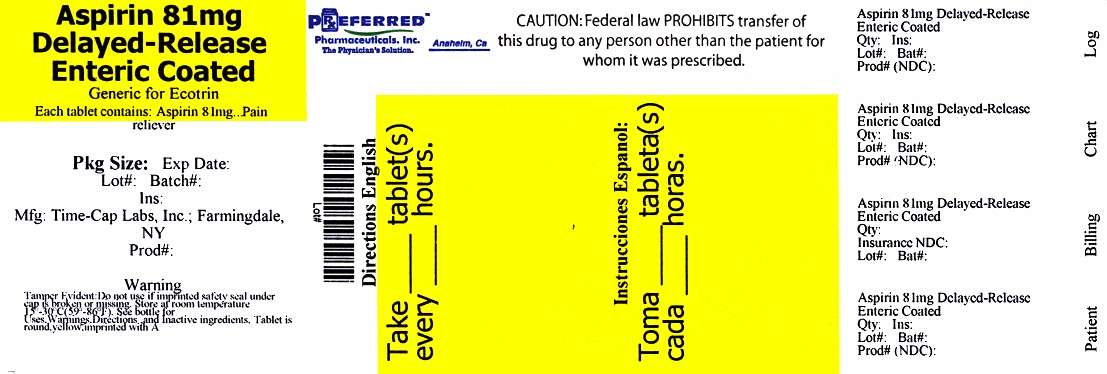
aspirin
Preferred Pharmaceuticals, Inc
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient in Each tablet
Aspirin 81 mg (NSAID) Non-steroidal anti-inflammatory drug
In each tablets Aspirin 81 mg (NSAID*)
Purpose
PAIN RELIEVER
Uses
INDICATIONS AND USAGE:
Pain Reliever – temporarily relieves minor aches and pains. For other uses, see your doctor. Do not use for more than 10 days without consulting your doctor because serious side effects may occur.
WARNINGS:
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product,, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include: facial swelling, shock, hives, asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinning (anticoagulant) or steroid drug, take other drugs containing prescription or nonprescription NSAIDS(aspirin, ibuprofen, naproxen or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed.
DOSAGE AND ADMINISTRATION:
Drink a full glass of water with each dose. Adults and children 12 years and over; take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours unless directed by a doctor. Children under 12 years; consult a doctor.
acetylated monoglycerides, anhydrous lactose, colloidal silicon dioxice, croscarmellose sodium, D-CYellow no.10 aluminum lake, hypromellose, hypromellose phthalate, iron oxide yellow (iron oxide ochre), microcrystalline cellulose, mineral oil, polyethylene glycol (PEG) 80, titantium dioxide
Keep Out of the Reach of Children: In case of overdose, get medical help or contact a Poison Control Center right away
How Supplied
Bottle of 120 - 68788-0186-2
Relabeled by Preferred Pharmaceuticals, Inc
Anaheim, CA 92870
aspirinaspirin TABLET, COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||