ariSulfur
ariSulfur Acne Treatment Soap
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Indications
- Warnings
- Directions
- Inactive Ingredients
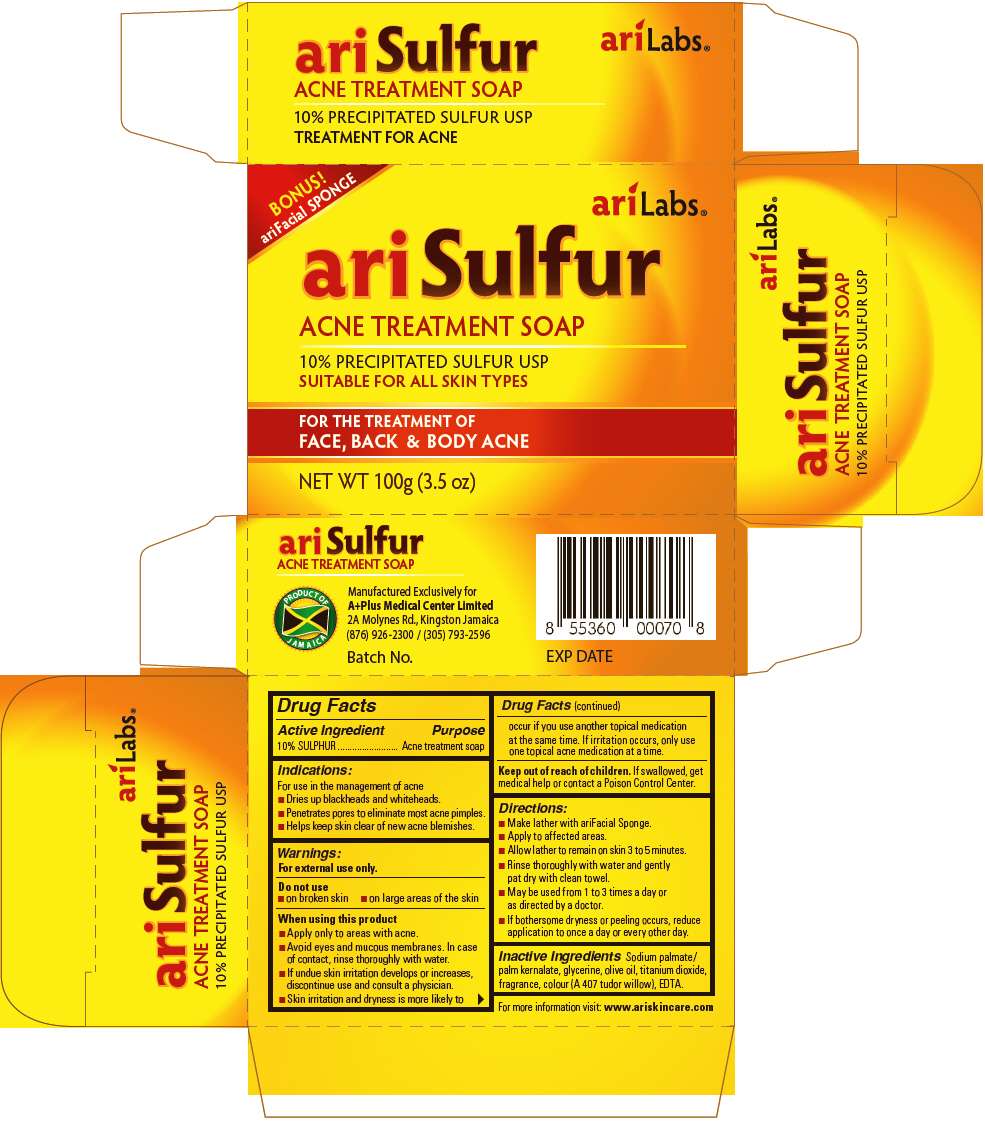
- PRINCIPAL DISPLAY PANEL - 100g Box
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
10% SULPHUR
Purpose
Acne Treatment Soap
Indications
For use in the management of acne
- Dries up blackheads and whiteheads.
- Penetrates pores to eliminate most acne pimples.
- Helps keep skin clear of new acne blemishes.
Warnings
For external use only.
Do not use on
- Broken skin
- Large areas of the skin
When using this product
- Apply only to areas with acne.
- Avoid eyes and mucous membranes. In case of contact, rinse thoroughly with water.
- If undue skin irritation develops or increases, discontinue use and consult a physician.
- Skin irritation and dryness is more likely to occur if you use another topical medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center.
Directions
- Make lather with ariFacial Sponge.
- Apply to affected areas.
- Allow lather to remain on skin 3 to 5 minutes.
- Rinse thoroughly with water and gently pat dry with clean towel.
- May be used from 1 to 3 times a day or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
SODIUM PALMATE / PALM KERNALATE, GLYCERINE, OLIVE OIL, TITANIUM DIOXIDE, FRAGRANCE, COLOUR (A 407 TUDOR WILLOW), EDTA.
For more information visit:
www.ariskincare.com
PRINCIPAL DISPLAY PANEL - 100g Box
BONUS!
ariFacial SPONGE
ariLabs ®
ariSulfur
ACNE TREATMENT SOAP
10% PRECIPITATED SULFUR USP
SUITABLE FOR ALL SKIN TYPES
FOR THE TREATMENT OF
FACE, BACK & BODY ACNE
NET WT 100g (3.5 oz)
ariSulfurSulfur SOAP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||