Apis compositum
Apis compositum Tablet
FULL PRESCRIBING INFORMATION: CONTENTS*
- KEEP OUT OF REACH OF CHILDREN
- APIS COMPOSITUM INDICATIONS AND USAGE
- WARNINGS
- APIS COMPOSITUM DOSAGE AND ADMINISTRATION
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
FULL PRESCRIBING INFORMATION
KEEP OUT OF REACH OF CHILDREN
Keep this and all medicines out of the reach of children.
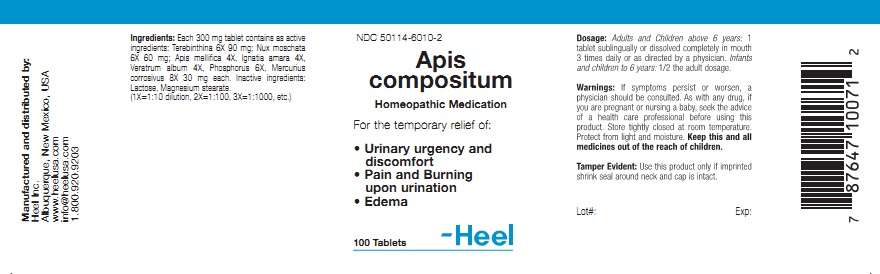
APIS COMPOSITUM INDICATIONS AND USAGE
For the temporary relief of:
- Urinary urgency and discomfort
- Pain and Burning upon urination
- Edema
WARNINGS
If symptoms persist
or worsen, a physician should be consulted. As with any drug, if you are
pregnant or nursing a baby, seek the advice of a health care professional before
using this product.
APIS COMPOSITUM DOSAGE AND ADMINISTRATION
Adults and children above 6 years: 1 tablet sublingually or dissolved completely in mouth 3 times daily, or as directed by a physician. Infants and children to 6 years: 1/2 the adult dosage.
ACTIVE INGREDIENT
Each 300 mg tablet contains as active ingredients: Terebinthia 6X 90 mg; Nux moschata 6X 60 mg; Apis melllifica 4X, Ignata amara 4X, Veratrum album 4X, Phosphorus 6X, Mercurius corrosivus 8X 30 mg each.
INACTIVE INGREDIENT
Lactose, Magnesium stearate
PURPOSE
Urinary urgency and discomfort, Pain and Burning upon urination, Edema
Apis compositumTURPENTINE OIL and NUTMEG and APIS MELLIFERA and STRYCHNOS IGNATII SEED and VERATRUM ALBUM ROOT and PHOSPHORUS and MERCURIC CHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||