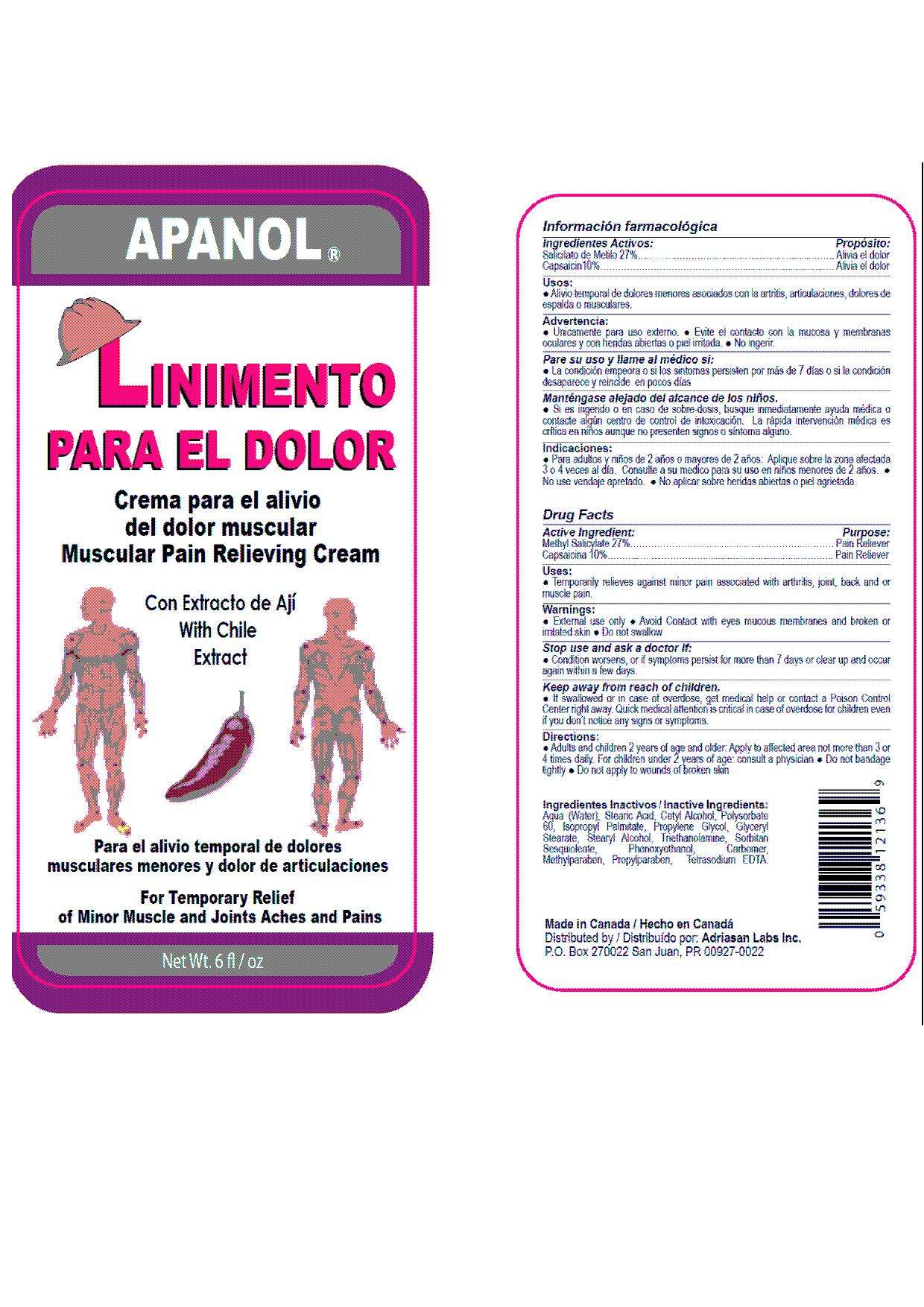
Apanol
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredient
Purpose
Pain Reliever, Pain Reliever
Apanol Uses
- Temporary relieves minor pain associated with arthritis, joint, back and or muscle pain.
Warnings
- External use only.
- Avoid contact with eyes, mucous membranes and broken or irritated skin.
- Do not swallow.
Stop use and ask doctor if
- condition worsens or if symptom persist for more than 7 days or clear up and occur again with a few days.
Keep away from reach of children
If swallowed or in case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical in case of overdose for children even if you don't notice any signs or symptoms.
Directions
- Adults and children 2 years of age and older: Apply to the affected area not more than 3 or 4 times daily. For children under 2 years of age: consult a physician.
- Do not bandage tightly.
- Do not apply to wounds of broken skin.
Inactive Ingredients
Aqua (Water), Stearic Acid, Cetyl Alcohol, Polysorbate 60, Isopropyl Palmitate, Propylene Glycol, Glyceryl Stearate, Stearyl Alcohol, Triethanolamine, Sorbitan Sesquioleate, Phenoxyethanol, Carbomer, Methylparaben, Propylparaben, Tetrasodium EDTA.
 mm1
mm1
ApanolMethyl Salicylate, Capsaicin OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!