Anti-bacterial Hand
Jets, Sets, & Elephants Beauty Corp.
bath-body-etc anti-bacterial hand gel - Sweet Cherry Blossom, Warm Vanilla Sugar, Vanilla Bean Noel, Merry Berry, Peppermint Twist, Kiss and Tell, Spring Fling, Sweet Cherry Blossom, Spring Blossom
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
- DIRECTIONS
- Inactive Ingredients
- QUESTIONS?
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
FULL PRESCRIBING INFORMATION
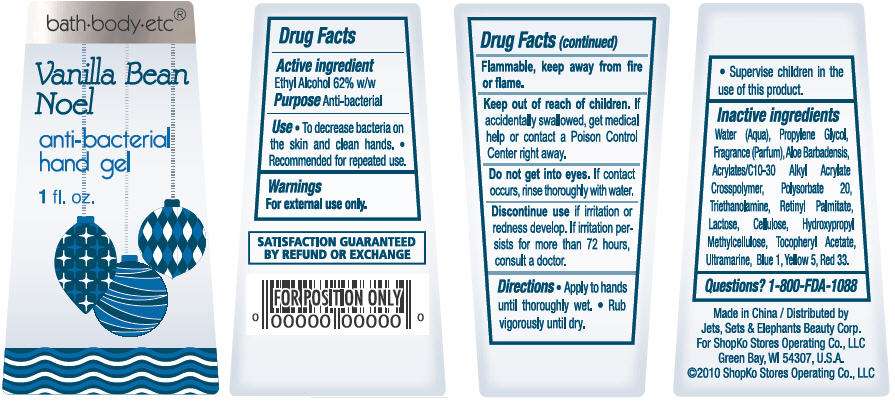
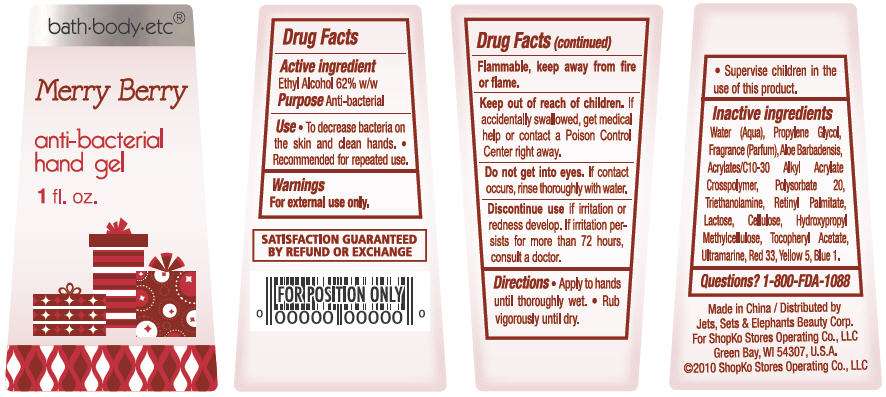
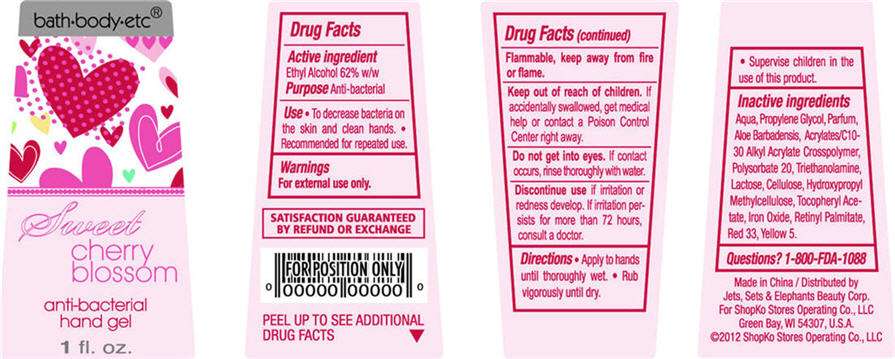
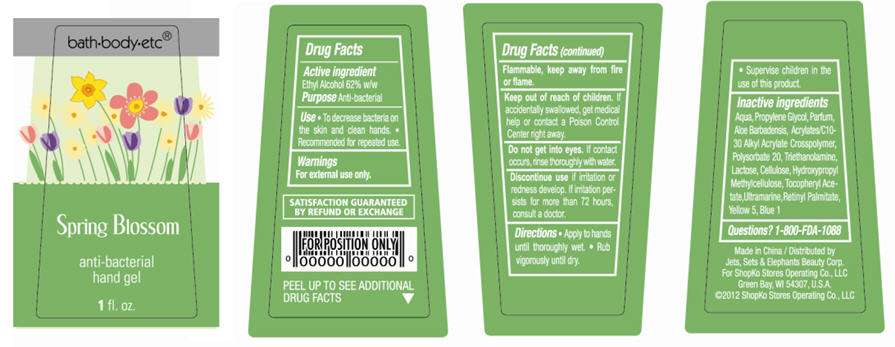
ACTIVE INGREDIENT
Ethyl Alcohol 62% w/w
PURPOSE
Anti-bacterial
USE
- To decrease bacteria on the skin and clean hands.
- Recommended for repeated use.
WARNINGS
For external use only. Flammable, keep away from fire or flame.
Keep out of reach of children.
If accidentally swallowed, get medical help or contact a Poison Control Center right away.
Do not get into eyes.
If contact occurs, rinse thoroughly with water.
Discontinue use
if irritation or redness develop. If irritation persists for more than 72 hours, consult a doctor.
DIRECTIONS
- Apply to hands until thoroughly wet.
- Rub vigorously until dry.
- Supervise children in the use of this product.
Inactive Ingredients
Sweet Cherry Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Warm Vanilla Sugar
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5, Blue 1
Vanilla Bean Noel
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Merry Berry
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Peppermint Twist
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Kiss and Tell
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Polysorbate 20, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Spring Fling
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Polysorbate 20, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Chromium Hydroxide Green, Retinyl Palmitate, Yellow 5, Blue 1
Sweet Cherry Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Spring Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Ultramarine, Retinyl Palmitate, Yellow 5, Blue 1
QUESTIONS?
1-800-FDA-1088
Made in China
Distributed by Jets, Sets & Elephants Beauty Corp.
For ShopKo Stores Operating Co., LLC
Green Bay, WI 54307, U.S.A
©2010 ShopKo Stores Operating Co., LLC
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Sweet Cherry Blossom
anti-bacterial
hand gel
PARABEN FREE
contains aloe vera and
vitamins A & E
1 fl oz

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Warm Vanilla Sugar
anti-bacterial
hand gel
PARABEN FREE
contains aloe vera and
vitamins A & E
1 fl oz

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Vanilla Bean Noel
anti-bacterial
hand gel
1 fl oz
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Merry Berry
anti-bacterial
hand gel
1 fl oz
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Peppermint Twist
anti-bacterial
hand gel
1 fl oz

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Kiss and tell
anti-bacterial
hand gel
1 fl oz

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Spring fling
anti-bacterial
hand gel
1 fl oz

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Sweet cherry blossom
anti-bacterial
hand gel
1 fl oz
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Spring Blossom
anti-bacterial
hand gel
1 fl oz
Anti-bacterial HandEthyl Alcohol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anti-bacterial HandEthyl Alcohol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||