Andalou BB Untinted with SPF-30
Andalou BB Untinted with SPF-30
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Andalou BB Untinted with SPF-30 Uses
- Warnings
- Directions
- Inactive Ingredients
- Other Information
- Questions
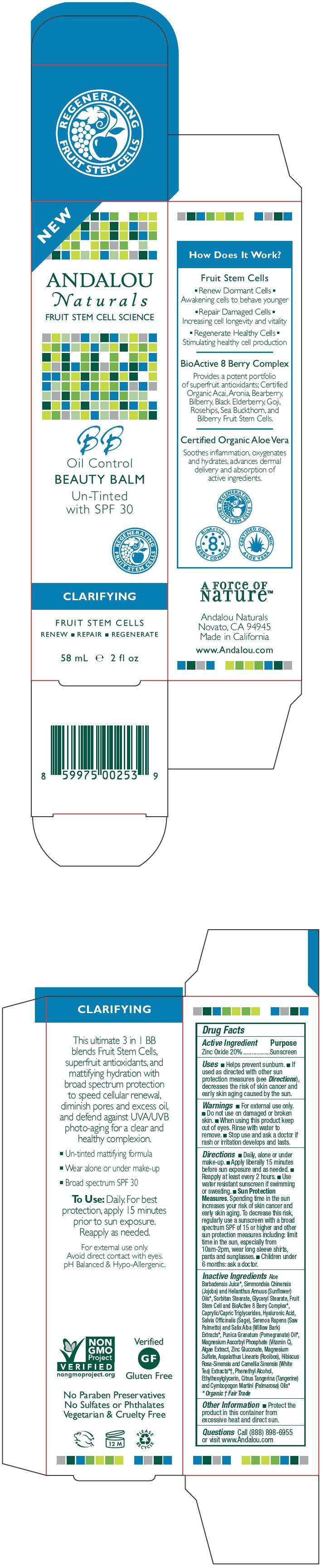
- PRINCIPAL DISPLAY PANEL - 58 mL Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Zinc Oxide 20%
Purpose
Sunscreen
Andalou BB Untinted with SPF-30 Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions ), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
- For external use only.
- Do not use on damaged or broken skin.
- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash or irritation develops and lasts.
Directions
- Daily, alone or under make-up.
- Apply liberally 15 minutes before sun exposure and as needed.
- Reapply at least every 2 hours.
- Use water resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10am-2pm, wear long sleeve shirts, pants and sunglasses.
- Children under 6 months: ask a doctor.
Inactive Ingredients
Aloe Barbadensis Juice





Other Information
- Protect the product in this container from excessive heat and direct sun.
Questions
Call (888) 898-6955 or visit www.Andalou.com
PRINCIPAL DISPLAY PANEL - 58 mL Tube Carton
NEW
ANDALOU
Naturals
FRUIT STEM CELL SCIENCE
BB
Oil Control
BEAUTY BALM
Un-Tinted
with SPF 30
REGENERATING
FRUIT STEM CELLS
CLARIFYING
FRUIT STEM CELLS
RENEW ■ REPAIR ■ REGENERATE
58 mL e 2 fl oz
Andalou BB Untinted with SPF-30Zinc Oxide LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||