AMOXICILLIN
STAT Rx USA LLC
PSS World Medical Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use amoxicillin capsules safely and effectively. See full prescribing information for amoxicillin capsules, USP. Amoxicillin Capsules, USPInitial U.S. Approval: 1974 MicrobiologyTo reduce the development of drug-resistant bacteria and maintain the effectiveness of amoxicillin capsules, USP and other antibacterial drugs, amoxicillin capsules, USP should be used only to treat infections that are proven or strongly suspected to be caused by bacteria.INDICATIONS AND USAGE Infections of the ear, nose, throat, genitourinary tract, skin and skin structure, and lower respiratory tract. (1.1, 1.2, 1.3, 1.4, 1.5) In combination for treatment of H. pylori infection and duodenal ulcer disease. (1.6, 1.7) DOSAGE AND ADMINISTRATION In adults, 750 to 1750 mg/day in divided doses every 8 to 12 hours. In Pediatric Patients > 3 Months of Age, 20 to 45 mg/kg/day in divided doses every 8 to 12 hours. Refer to full prescribing information for specific dosing regimens. (2.1, 2.2, 2.3) Treatment of gonorrhea is 3 grams as a single oral dose. (2.1) The upper dose for neonates and infants ≤ 3 months is 30 mg/kg/day divided every 12 hours. (2.2) Dosing for H. pylori Infection: Triple Therapy: 1 gram amoxicillin, 500 mg clarithromycin, and 30 mg lansoprazole, all given twice daily (every 12 hours) for 14 days. Dual Therapy: 1 gram amoxicillin and 30 mg lansoprazole, each given three times daily (every 8 hours) for 14 days. (2.3) Reduce the dose in patients with severe renal impairment (GFR
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 AMOXICILLIN INDICATIONS AND USAGE
- 1.1 Infections of the Ear, Nose, and Throat
- 1.2 Infections of the Genitourinary Tract
- 1.3 Infections of the Skin and Skin Structure
- 1.4 Infections of the Lower Respiratory Tract
- 1.5 Gonorrhea, Acute Uncomplicated (ano-genital and urethral infections in males and females)
- 1.6 Triple Therapy for Helicobacter pylori with Clarithromycin and Lansoprazole
- 1.7 Dual Therapy for H. pylori with Lansoprazole
- 2 AMOXICILLIN DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 AMOXICILLIN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 AMOXICILLIN ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 AMOXICILLIN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL - AMOXICILLIN - 250 mg CAPSULES
- PACKAGE LABEL - AMOXICILLIN - 500 mg CAPSULES
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Infections of the Ear, Nose, and Throat
StreptococcusStreptococcus pneumoniaeStaphylococcusHaemophilus influenzae
1.2 Infections of the Genitourinary Tract
Escherichia coli, Proteus mirabilisEnterococcus faecalis
1.3 Infections of the Skin and Skin Structure
StreptococcusStaphylococcusE. coli
1.4 Infections of the Lower Respiratory Tract
StreptococcusS. pneumoniae, StaphylococcusH. influenzae
1.5 Gonorrhea, Acute Uncomplicated (ano-genital and urethral infections in males and females)
Neisseria gonorrhoeae
N. gonorrhoeae
1.6 Triple Therapy for Helicobacter pylori with Clarithromycin and Lansoprazole
H. pyloriH. pyloriH. pylori
1.7 Dual Therapy for H. pylori with Lansoprazole
H. pyloriwho are either allergic or intolerant to clarithromycin or in whom resistance to clarithromycin is known or suspectedH. pylori
2 DOSAGE AND ADMINISTRATION
2.1 Dosing for Adult and Pediatric Patients > 3 Months of Age
Streptococcus pyogenes
| Infection | Severitya | Usual Adult Dose | Usual Dose for Children > 3 Monthsb |
|---|---|---|---|
|
a Dosing for infections caused by bacteria that are intermediate in their susceptibility to amoxicillin should follow the recommendations for severe infections. b The children’s dosage is intended for individuals whose weight is less than 40 kg. Children weighing 40 kg or more should be dosed according to the adult recommendations. |
|||
| Ear/Nose/Throat Skin/Skin Structure Genitourinary Tract |
Mild/Moderate |
500 mg every 12 hours or 250 mg every 8 hours |
25 mg/kg/day in divided doses every 12 hours or 20 mg/kg/day in divided doses every 8 hours |
| Severe |
875 mg every 12 hours or 500 mg every 8 hours |
45 mg/kg/day in divided doses every 12 hours or 40 mg/kg/day in divided doses every 8 hours |
|
| Lower Respiratory Tract |
Mild/Moderate or Severe |
875 mg every 12 hours or 500 mg every 8 hours |
45 mg/kg/day in divided doses every 12 hours or 40 mg/kg/day in divided doses every 8 hours |
| Gonorrhea Acute, uncomplicated ano -genital and urethral infections in males and females |
|
3 grams as single oral dose |
Prepubertal children: 50 mg/kg amoxicillin capsules, combined with 25 mg/kg probenecid as a single dose. Note: Since probenecid is contraindicated in children under 2 years, do not use this regimen in children under 2 years of age. |
2.2 Dosing in Neonates and Infants Aged ≤ 12 Weeks (≤ 3 Months)
Streptococcus pyogenes
2.3 Dosing for H. pylori Infection
Triple Therapy:
Dual Therapy:
2.4 Dosing in Renal Impairment
- Patients with impaired renal function do not generally require a reduction in dose unless the impairment is severe.
- Severely impaired patients with a glomerular filtration rate of < 30 mL/min should not receive a 875 mg dose.
- Patients with a glomerular filtration rate of 10 to 30 mL/min should receive 500 mg or 250 mg every 12 hours, depending on the severity of the infection.
- Patients with a glomerular filtration rate less than 10 mL/min should receive 500 mg or 250 mg every 24 hours, depending on severity of the infection.
- Hemodialysis patients should receive 500 mg or 250 mg every 24 hours, depending on severity of the infection. They should receive an additional dose both during and at the end of dialysis.
3 DOSAGE FORMS AND STRENGTHS
250 mg Capsule
500 mg Capsule
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylactic Reactions
5.2 Clostridium difficile Associated Diarrhea
Clostridium difficile C. difficile. C. difficile C. difficile
C. difficileC. difficile
5.3 Potential for Microbial Overgrowth or Bacterial Resistance
5.4 Use in Patients With Mononucleosis
6 ADVERSE REACTIONS
- Anaphylactic reactions [see Warnings and Precautions (5.1)]
- CDAD [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Triple Therapy:
Dual Therapy:
6.2 Postmarketing or Other Experience
- Infections and Infestations: Mucocutaneous candidiasis.
- Gastrointestinal: Black hairy tongue, and hemorrhagic/pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment [see Warnings and Precautions (5.2)].
- Hypersensitivity Reactions: Anaphylaxis [see Warnings and Precautions (5.1)]. Serum sickness–like reactions, erythematous maculopapular rashes, erythema multiforme, Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, hypersensitivity vasculitis, and urticaria have been reported.
- Liver: A moderate rise in AST and/or ALT has been noted, but the significance of this finding is unknown. Hepatic dysfunction including cholestatic jaundice, hepatic cholestasis and acute cytolytic hepatitis have been reported.
- Renal: Crystalluria has been reported [see Overdosage (10)].
- Hemic and Lymphatic Systems: Anemia, including hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis have been reported. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
- Central Nervous System: Reversible hyperactivity, agitation, anxiety, insomnia, confusion, convulsions, behavioral changes, and/or dizziness have been reported.
- Miscellaneous: Tooth discoloration (brown, yellow, or gray staining) has been reported. Most reports occurred in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases.
7 DRUG INTERACTIONS
7.1 Probenecid
7.2 Oral Anticoagulants
7.3 Allopurinol
7.4 Oral Contraceptives
7.5 Other Antibacterials
in vitro
7.6 Effects on Laboratory Tests
®®
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects:
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
[See Dosage and Administration (2.2).]
8.5 Geriatric Use
8.6 Dosing in Renal Impairment
See Dosage and Administration (2.4)
10 OVERDOSAGE
1
11 DESCRIPTION
SRRRp
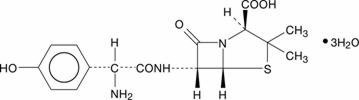
1619352
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
[see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Absorption:
0-∞ max
| Dose * | AUC0-∞ (mcg●hr/mL) | Cmax (mcg/mL)† |
|---|---|---|
|
* Administered at the start of a light meal. † Mean values of 24 normal volunteers. Peak concentrations occurred approximately 1 hour after the dose. |
||
| Amoxicillin |
Amoxicillin (±S.D.) |
Amoxicillin (±S.D.) |
| 400 mg (5 mL of suspension) |
17.1 (3.1) |
5.92 (1.62) |
| 400 mg (1 chewable tablet) |
17.9 (2.4) |
5.18 (1.64) |
Distribution:
Metabolism and Excretion:[see Drug Interactions (7.1)]
12.4 Microbiology
Mechanism of Action
Method of Resistance
in vitro INDICATIONS AND USAGE
|
Gram-Positive Bacteria
|
Gram-Negative Bacteria
|
|
Enterococcus faecalis
Staphylococcus spp. Streptococcus pneumoniae Alpha and β-hemolytic streptococci. |
Escherichia coli
Haemophilus influenzae Neisseria gonorrhoeae Proteus mirabilis Helicobacter pylori |
Susceptibility Test Methods:
in vitro
Dilution Techniques: 2,3
Diffusion Techniques: 3
| Minimum Inhibitory Concentration (mcg/mL) |
Disk Diffusion (zone diameter in mm) |
|||||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| * S. pneumoniae should be tested using a 1 mcg oxacillin disk. Isolates with oxacillin zone sizes of ≥ 20 mm are susceptible to amoxicillin. An amoxicillin MIC should be determined on isolates of S. pneumoniae with oxacillin zone sizes of ≤ 19 mm. **A positive beta lactamase test indicates resistance to amoxicillin. Isolates that are resistant to penicillin by MIC testing are also expected to be resistant to amoxicillin. |
||||||
|
Enterococcus spp. |
≤ 8 |
- |
≥ 16 |
≥ 17 |
- |
≤ 16 |
|
Staphylococcus spp. |
≤ 0.25 |
≥ 0.5 |
≥ 29 |
≤ 28 |
||
| Streptococci, viridians group (alpha-hemolytic streptococci) |
≤ 0.25 |
0.5 to 4 |
≥ 8 |
- |
- |
- |
| β-hemolytic streptococci |
≤ 0.25 |
- |
- |
≥ 24 |
- |
- |
|
Streptococcus pneumoniae
(non-meningitis isolates)* |
≤ 2 |
4 |
≥ 8 |
- |
- |
- |
|
Enterobacteriaceae
|
≤ 8 |
16 |
≥ 32 |
≥ 17 |
14 to 16 |
≤ 13 |
|
Haemophilus influenzae
|
≤ 1 |
2 |
≥ 4 |
≥ 22 |
19 to 21 |
≤ 18 |
|
Neisseria gonorrhoeae**
|
- |
- |
- |
- |
- |
- |
Quality Control
2,3,4
| Bacteria | ATCC# | MIC Range (mcg/mL) |
Disk Diffusion Zone Range (mm) |
|---|---|---|---|
|
# ATCC = American Type Culture Collection |
|||
|
Escherichia coli
|
25922 |
2 to 8 |
16 to 22 |
|
Enterococcus faecalis
|
29212 |
0.5 to 2 |
|
|
Haemophilus influenzae
|
49247 |
2 to 8 |
13 to 21 |
|
Staphylococcus aureus
|
29213 |
0.5 to 2 |
|
| 25923 |
27 to 35 |
||
|
Streptococcus pneumoniae
|
49619 |
0.06 to 0.25 |
|
Susceptibility Testing for Helicobacter pylori: Amoxicillin in vitro susceptibility testing methods for determining minimum inhibitory concentrations (MICs) and zone sizes have not been standardized, validated, or approved for testing H. pylori. Specimens for H. pylori and clarithromycin susceptibility test results should be obtained on isolates from patients who fail triple therapy. If clarithromycin resistance is found, a non-clarithromycin-containing regimen should be used.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
H. pyloriH. pylori
Triple Therapy:
Dual Therapy:H. pyloriH. pylori
| Study | Triple Therapy | Triple Therapy |
|---|---|---|
| Evaluable Analysisa
[95% Confidence Interval] (number of patients) |
Intent-to-Treat Analysisb
[95% Confidence Interval] (number of patients) |
|
|
a This analysis was based on evaluable patients with confirmed duodenal ulcer (active or within 1 year) and H. pylori infection at baseline defined as at least 2 of 3 positive endoscopic tests from CLOtest®, histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy. b Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within 1 year). All dropouts were included as failures of therapy. |
||
| Study 1 |
92 [80 - 97.7] (n = 48) |
86 [73.3 - 93.5] (n = 55) |
| Study 2 |
86 [75.7 - 93.6] (n = 66) |
83 [72 - 90.8] (n = 70) |
| Study | Dual Therapy | Dual Therapy |
|---|---|---|
| Evaluable Analysisa
[95% Confidence Interval] (number of patients) |
Intent-to-Treat Analysisb
[95% Confidence Interval] (number of patients) |
|
|
a This analysis was based on evaluable patients with confirmed duodenal ulcer (active or within 1 year) and H. pylori infection at baseline defined as at least 2 of 3 positive endoscopic tests from CLOtest®, histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy. b Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within 1 year). All dropouts were included as failures of therapy. |
||
| Study 1 |
77 [62.5 - 87.2] (n = 51) |
70 [56.8 - 81.2] (n = 60) |
| Study 2 |
66 [51.9 - 77.5] (n = 58) |
61 [48.5 - 72.9] (n = 67) |
15 REFERENCES
- Swanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988; 30: 66-67.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – 8th ed. CLSI Document M7-A8, Vol. 29, No.2. CLSI, Wayne, PA, Jan. 2009.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standard for Antimicrobial Disk Susceptibility Tests; Approved Standard – 10th ed. CLSI Document M2-A10, Vol. 29, No. 1. CLSI, Wayne, PA, 2009.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: 21st Informational Supplement. Approved Standard CLSI Document M100-S21 CLSI, Wayne, PA, January 2011.
16 HOW SUPPLIED/STORAGE AND HANDLING
Amoxicillin Capsules, USP
250 mg Capsule
500 mg Capsule
Store at
Relabeling and Repackaging by
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
- Patients should be advised that amoxicillin may be taken every 8 hours or every 12 hours, depending on the dose prescribed.
- Patients should be counseled that antibacterial drugs, including amoxicillin, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When amoxicillin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by amoxicillin or other antibacterial drugs in the future.
- Patients should be counseled that diarrhea is a common problem caused by antibiotics, and it usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having taken their last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
- Patients should be aware that amoxicillin contains a penicillin class drug product that can cause allergic reactions in some individuals.
®
®
®
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL - AMOXICILLIN - 250 mg CAPSULES
Amoxicillin Capsules, USP 250 mg
Rx only

PACKAGE LABEL - AMOXICILLIN - 500 mg CAPSULES
Amoxicillin Capsules, USP 500 mg
Rx only

AMOXICILLINAMOXICILLIN CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AMOXICILLINAMOXICILLIN CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!