Amitriptyline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- AMITRIPTYLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- AMITRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PREGNANCY
- NURSING MOTHERS
- PRECAUTIONS
- PEDIATRIC USE
- GERIATRIC USE
- AMITRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsWarnings: Clinical Worsening and Suicide RiskPrecautions: Information for PatientsPrecautions: Pediatric Use
AMITRIPTYLINE HYDROCHLORIDE DESCRIPTION
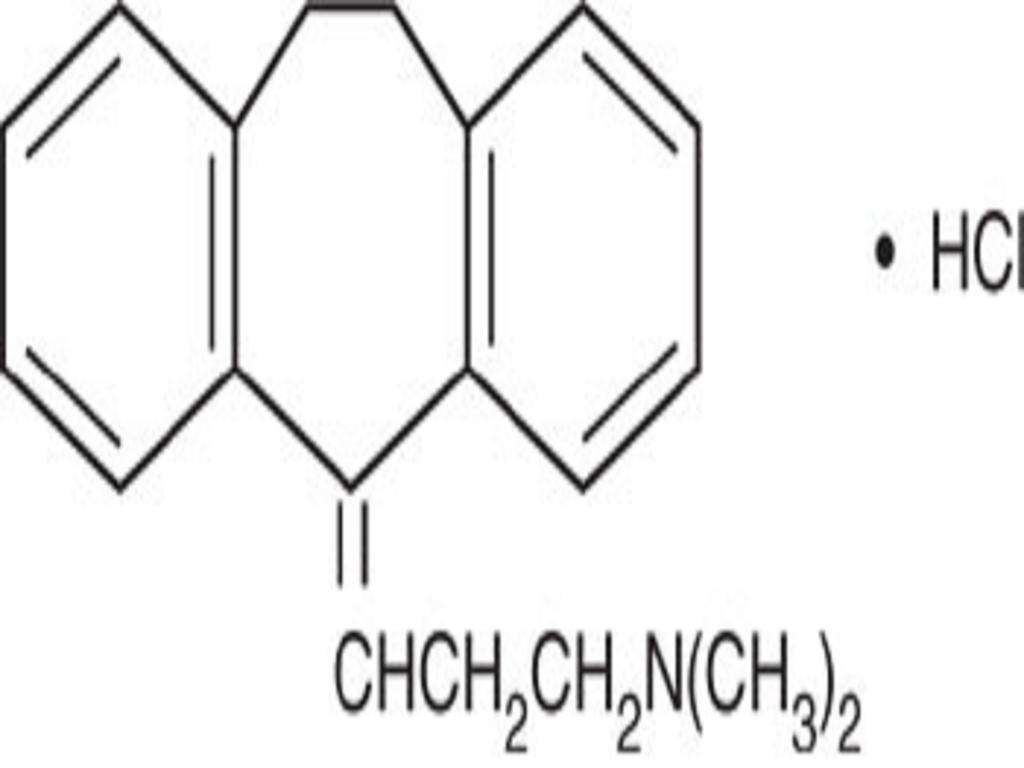
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
AMITRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide Risk:Screening Patients for Bipolar Disorder:
PREGNANCY
Pregnancy Category CNURSING MOTHERS
PRECAUTIONS
Information for Patients:
Medication Guide"Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions"
Clinical Worsening and Suicide Risk:
Drug Interactions:
Drugs Metabolized by P450 2D6
Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be coadministered with another drug known to be an inhibitor of P450 2D6.
Monoamine oxidase inhibitors
CONTRAINDICATIONSseeWARNINGS
PEDIATRIC USE
BOX WARNINGWARNINGS-Clinical Worsening and Suicide RiskGERIATRIC USE
DOSAGE AND ADMINISTRATION
AMITRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
OVERDOSAGE
Gastrointestinal Decontamination:
Cardiovascular:
CNS:
Psychiatric Follow-up:
Pediatric Management:
DOSAGE & ADMINISTRATION
Initial Dosage for Adults:
Adolescent and Elderly Patients:
Maintenance:
Usage in Pediatric Patients
Plasma Levels
HOW SUPPLIED
STORAGE AND HANDLING
METABOLISM
REFERENCES
SPL MEDGUIDE
Antidepressant Medicines, Depression and Other Serious Mental llnesses, and Suicidal Thoughts or Actions
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
● What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● What else do I need to know about antidepressant medicines?
-
● Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
-
● Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Amitriptyline HydrochlorideAmitriptyline Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!